Long Prime-Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine
- PMID: 38932316
- PMCID: PMC11209353
- DOI: 10.3390/vaccines12060587
Long Prime-Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine
Abstract
The carbohydrate ganglioside GD2/GD3 cancer vaccine adjuvanted by β-glucan stimulates anti-GD2 IgG1 antibodies that strongly correlate with improved progression-free survival (PFS) and overall survival (OS) among patients with high-risk neuroblastoma. Thirty-two patients who relapsed on the vaccine (first enrollment) were re-treated on the same vaccine protocol (re-enrollment). Titers during the first enrollment peaked by week 32 at 751 ± 270 ng/mL, which plateaued despite vaccine boosts at 1.2-4.5 month intervals. After a median wash-out interval of 16.1 months from the last vaccine dose during the first enrollment to the first vaccine dose during re-enrollment, the anti-GD2 IgG1 antibody rose to a peak of 4066 ± 813 ng/mL by week 3 following re-enrollment (p < 0.0001 by the Wilcoxon matched-pairs signed-rank test). Yet, these peaks dropped sharply and continually despite repeated boosts at 1.2-4.5 month intervals, before leveling off by week 20 to the first enrollment peak levels. Despite higher antibody titers, patients experienced no pain or neuropathic side effects, which were typically associated with immunotherapy using monoclonal anti-GD2 antibodies. By the Kaplan-Meier method, PFS was estimated to be 51%, and OS was 81%. The association between IgG1 titer during re-enrollment and β-glucan receptor dectin-1 SNP rs3901533 was significant (p = 0.01). A longer prime-boost interval could significantly improve antibody responses in patients treated with ganglioside conjugate cancer vaccines.
Keywords: anti-GD2 IgG1 titer; beta-glucan; dectin-1 SNP rs3901533; ganglioside GD2/GD3 carbohydrate vaccine; high-risk neuroblastoma.
Conflict of interest statement
N.K.C. is the inventor and owner of issued patents licensed by Memorial Sloan Kettering Cancer Center (MSK) to Y-mAbs Therapeutics, Biotec Pharmacon/Lallemand, and Abpro-labs. MSK and N.K.C. have a financial interest in Y-mAbs. N.K.C.reports receiving stock options from Eureka Therapeutics and Abpro-labs. N.K.C.is a member of the scientific advisory board of Eureka Therapeutics. N.K.C.and S.M. were named as inventors on a patent on glucan adjuvant filed by MSK. S.M. declares consulting roles with Y-mAbs Therapeutics, US WorldMeds, EUSA Pharma and Innervate RP, Inc. Carbohydrate ganglioside GD2/GD3 cancer vaccine has been licensed by Memorial Sloan Kettering Cancer Center (MSK) to Y-mAbs Therapeutics.
Figures

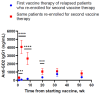

 ) to the last injection at re-enrollment (
) to the last injection at re-enrollment ( ). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.
). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.



Similar articles
-
Effect of Oral β-Glucan on Antibody Response to Ganglioside Vaccine in Patients With High-Risk Neuroblastoma: A Phase 2 Randomized Clinical Trial.JAMA Oncol. 2023 Feb 1;9(2):242-250. doi: 10.1001/jamaoncol.2022.5999. JAMA Oncol. 2023. PMID: 36547975 Free PMC article. Clinical Trial.
-
Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression.J Clin Oncol. 2021 Jan 20;39(3):215-226. doi: 10.1200/JCO.20.01892. Epub 2020 Dec 16. J Clin Oncol. 2021. PMID: 33326254 Free PMC article. Clinical Trial.
-
Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21.Clin Cancer Res. 2003 Nov 1;9(14):5214-20. Clin Cancer Res. 2003. PMID: 14614001
-
Anti-GD2 Directed Immunotherapy for High-Risk and Metastatic Neuroblastoma.Biomolecules. 2022 Feb 24;12(3):358. doi: 10.3390/biom12030358. Biomolecules. 2022. PMID: 35327550 Free PMC article. Review.
-
Anti-GD2 antibody-containing immunotherapy postconsolidation therapy for people with high-risk neuroblastoma treated with autologous haematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2019 Apr 24;4(4):CD012442. doi: 10.1002/14651858.CD012442.pub2. Cochrane Database Syst Rev. 2019. PMID: 31016728 Free PMC article.
References
-
- Kwong J.C., Chung H., Jung J.K., Buchan S.A., Campigotto A., Campitelli M.A., Crowcroft N.S., Gubbay J.B., Karnauchow T., Katz K., et al. The impact of repeated vaccination using 10-year vaccination history on protection against influenza in older adults: A test-negative design study across the 2010/11 to 2015/16 influenza seasons in Ontario, Canada. Eurosurveillance. 2020;25:1900245. doi: 10.2807/1560-7917.ES.2020.25.1.1900245. - DOI - PMC - PubMed
-
- Valenciano M., Kissling E., Larrauri A., Nunes B., Pitigoi D., O’Donnell J., Reuss A., Horváth J.K., Paradowska-Stankiewicz I., Rizzo C., et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: Results of the European I-MOVE multicentre test-negative case-control study, 2011/2012–2016/2017. Influenza Other Respir. Viruses. 2018;12:567–581. doi: 10.1111/irv.12562. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

