Risk of Seizure Aggravation after COVID-19 Vaccinations in Patients with Epilepsy
- PMID: 38932322
- PMCID: PMC11209536
- DOI: 10.3390/vaccines12060593
Risk of Seizure Aggravation after COVID-19 Vaccinations in Patients with Epilepsy
Abstract
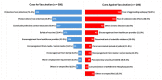
Although Coronavirus disease 2019 (COVID-19) vaccinations are generally recommended for persons with epilepsy (PwE), a significant vaccination gap remains due to patient concerns over the risk of post-vaccination seizure aggravation (PVSA). In this single-centre, retrospective cohort study, we aimed to determine the early (7-day) and delayed (30-day) risk of PVSA, and to identify clinical predictors of PVSA among PwE. Adult epilepsy patients aged ≥18 years without a history of COVID-19 infection were recruited from a specialty epilepsy clinic in early 2022. Demographic, epilepsy characteristics, and vaccination data were extracted from a centralized electronic patient record. Seizure frequency before and after vaccination, vaccination-related adverse effects, and reasons for or against vaccination were obtained by a structured questionnaire. A total of 786 PwEs were included, of which 27.0% were drug-resistant. At the time of recruitment, 74.6% had at least 1 dose of the COVID-19 vaccine. Subjects with higher seizure frequency (p < 0.0005), on more anti-seizure medications (p = 0.004), or had drug-resistant epilepsy (p = 0.001) were less likely to be vaccinated. No significant increase in seizure frequency was observed in the early (7 days) and delayed phases (30 days) after vaccination in our cohort. On the contrary, there was an overall significant reduction in seizure frequency 30 days after vaccination (1.31 vs. 1.89, t = 3.436; p = 0.001). This difference was seen in both types of vaccine (BNT162b2 and CoronaVac) and drug-resistant epilepsy, but just missed significance for the second dose (1.13 vs. 1.87, t = 1.921; p = 0.055). Only 5.3% had PVSA after either dose of vaccine. Higher pre-vaccination seizure frequency of ≥1 per week (OR 3.01, 95% CI 1.05-8.62; p = 0.04) and drug-resistant status (OR 3.32, 95% CI 1.45-249 7.61; p = 0.005) were predictive of PVSA. Meanwhile, seizure freedom for 3 months before vaccination was independently associated with a lower risk of PVSA (OR 0.11, 95% CI 0.04-0.28; p < 0.0005). This may guide epilepsy treatment strategies to achieve better seizure control for at least 3 months prior to vaccination. As COVID-19 shifts to an endemic phase, this study provides important data demonstrating the overall safety of COVID-19 vaccinations among PwE. Identification of high-risk patients with subsequent individualized approaches in treatment and monitoring strategies may alleviate vaccination hesitancy among PwE.
Keywords: COVID-19 infection; COVID-19 vaccine; epilepsy; neurological adverse effects; seizure; vaccination gap.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study.Seizure. 2021 Nov;92:2-9. doi: 10.1016/j.seizure.2021.08.001. Epub 2021 Aug 6. Seizure. 2021. PMID: 34391030 Free PMC article.
-
COVID-19 vaccination hesitancy and safety among adult people with epilepsy in eastern China.Epilepsy Behav. 2023 Jan;138:108984. doi: 10.1016/j.yebeh.2022.108984. Epub 2022 Nov 7. Epilepsy Behav. 2023. PMID: 36423385 Free PMC article.
-
Effect of inactivated COVID-19 vaccines on seizure frequency in patients with epilepsy: A multicenter, prospective study.Front Immunol. 2022 Dec 7;13:984789. doi: 10.3389/fimmu.2022.984789. eCollection 2022. Front Immunol. 2022. PMID: 36569941 Free PMC article.
-
COVID-19 vaccination for patients with epilepsy: A Chinese expert consensus.Epilepsy Behav. 2023 Oct;147:109387. doi: 10.1016/j.yebeh.2023.109387. Epub 2023 Aug 23. Epilepsy Behav. 2023. PMID: 37625346 Review.
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
Cited by
-
Epilepsy Care in the Real World of Multiple Medical Considerations.Epilepsy Curr. 2025 Jul 2:15357597251320144. doi: 10.1177/15357597251320144. Online ahead of print. Epilepsy Curr. 2025. PMID: 40621489 Free PMC article. Review.
-
Brain tumors and fitness to drive: A review and multi-disciplinary approach.Neurooncol Pract. 2024 Dec 6;12(2):183-196. doi: 10.1093/nop/npae119. eCollection 2025 Apr. Neurooncol Pract. 2024. PMID: 40110067 Review.
References
-
- Mohammed I., Nauman A., Paul P., Ganesan S., Chen K.-H., Jalil S.M.S., Jaouni S.H., Kawas H., Khan W.A., Vattoth A.L., et al. The Efficacy and Effectiveness of the COVID-19 Vaccines in Reducing Infection, Severity, Hospitalization, and Mortality: A Systematic Review. Hum. Vaccines Immunother. 2022;18:2027160. doi: 10.1080/21645515.2022.2027160. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous