A Unique mRNA Vaccine Elicits Protective Efficacy against the SARS-CoV-2 Omicron Variant and SARS-CoV
- PMID: 38932334
- PMCID: PMC11209356
- DOI: 10.3390/vaccines12060605
A Unique mRNA Vaccine Elicits Protective Efficacy against the SARS-CoV-2 Omicron Variant and SARS-CoV
Abstract
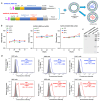
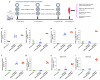
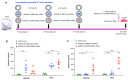
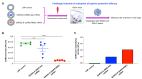
The highly pathogenic coronaviruses SARS-CoV-2 and SARS-CoV have led to the COVID-19 pandemic and SARS outbreak, respectively. The receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2, particularly the Omicron variant, has frequent mutations, resulting in the reduced efficiency of current COVID-19 vaccines against new variants. Here, we designed two lipid nanoparticle-encapsulated mRNA vaccines by deleting the mutant RBD of the SARS-CoV-2 Omicron variant (SARS2-S (RBD-del)) or by replacing this mutant RBD with the conserved and potent RBD of SARS-CoV (SARS2-S (SARS-RBD)). Both mRNA vaccines were stable at various temperatures for different time periods. Unlike SARS2-S (RBD-del) mRNA, SARS2-S (SARS-RBD) mRNA elicited effective T-cell responses and potent antibodies specific to both SARS-CoV-2 S and SARS-CoV RBD proteins. It induced strong neutralizing antibodies against pseudotyped SARS-CoV-2 and SARS-CoV infections and protected immunized mice from the challenge of the SARS-CoV-2 Omicron variant and SARS-CoV by significantly reducing the viral titers in the lungs after Omicron challenge and by completely preventing SARS-CoV-induced weight loss and death. SARS2-S (SARS-RBD)-immunized serum antibodies protected naïve mice from the SARS-CoV challenge, with its protective efficacy positively correlating with the neutralizing antibody titers. These findings indicate that this mRNA vaccine has the potential for development as an effective vaccine against current and future SARS-CoV-2 variants and SARS-CoV.
Keywords: COVID-19; SARS-CoV; SARS-CoV-2; coronavirus; receptor-binding domain; spike protein; unique mRNA vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Pan-beta-coronavirus subunit vaccine prevents SARS-CoV-2 Omicron, SARS-CoV, and MERS-CoV challenge.J Virol. 2024 Sep 17;98(9):e0037624. doi: 10.1128/jvi.00376-24. Epub 2024 Aug 27. J Virol. 2024. PMID: 39189731 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV.Vaccines (Basel). 2025 Mar 10;13(3):293. doi: 10.3390/vaccines13030293. Vaccines (Basel). 2025. PMID: 40266218 Free PMC article.
-
A mosaic-type trimeric RBD-based COVID-19 vaccine candidate induces potent neutralization against Omicron and other SARS-CoV-2 variants.Elife. 2022 Aug 25;11:e78633. doi: 10.7554/eLife.78633. Elife. 2022. PMID: 36004719 Free PMC article.
-
Universal subunit vaccine protects against multiple SARS-CoV-2 variants and SARS-CoV.NPJ Vaccines. 2024 Jul 25;9(1):133. doi: 10.1038/s41541-024-00922-z. NPJ Vaccines. 2024. PMID: 39054338 Free PMC article.
References
-
- World Health Organization Coronavirus (COVID-19) Dashboard. 2023. [(accessed on 28 April 2024)]. Available online: https://covid19.who.int/
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

