Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients: The RIVASTIM-Inulin Randomised Controlled Trial
- PMID: 38932337
- PMCID: PMC11209582
- DOI: 10.3390/vaccines12060608
Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients: The RIVASTIM-Inulin Randomised Controlled Trial
Abstract
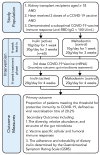
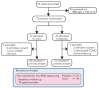
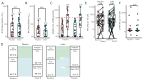
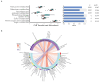
Kidney transplant recipients are at an increased risk of hospitalisation and death from SARS-CoV-2 infection, and standard two-dose vaccination schedules are typically inadequate to generate protective immunity. Gut dysbiosis, which is common among kidney transplant recipients and known to effect systemic immunity, may be a contributing factor to a lack of vaccine immunogenicity in this at-risk cohort. The gut microbiota modulates vaccine responses, with the production of immunomodulatory short-chain fatty acids by bacteria such as Bifidobacterium associated with heightened vaccine responses in both observational and experimental studies. As SCFA-producing populations in the gut microbiota are enhanced by diets rich in non-digestible fibre, dietary supplementation with prebiotic fibre emerges as a potential adjuvant strategy to correct dysbiosis and improve vaccine-induced immunity. In a randomised, double-bind, placebo-controlled trial of 72 kidney transplant recipients, we found dietary supplementation with prebiotic inulin for 4 weeks before and after a third SARS-CoV2 mRNA vaccine to be feasible, tolerable, and safe. Inulin supplementation resulted in an increase in gut Bifidobacterium, as determined by 16S RNA sequencing, but did not increase in vitro neutralisation of live SARS-CoV-2 virus at 4 weeks following a third vaccination. Dietary fibre supplementation is a feasible strategy with the potential to enhance vaccine-induced immunity and warrants further investigation.
Keywords: COVID-19; SARS-CoV-2; dysbiosis; immunisation; immunity; kidney transplantation; microbiota; prebiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rapamycin and inulin for third-dose vaccine response stimulation (RIVASTIM): Inulin - study protocol for a pilot, multicentre, randomised, double-blinded, controlled trial of dietary inulin to improve SARS-CoV-2 vaccine response in kidney transplant recipients.BMJ Open. 2022 Dec 1;12(12):e062747. doi: 10.1136/bmjopen-2022-062747. BMJ Open. 2022. PMID: 36456021 Free PMC article.
-
Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients.Trials. 2022 Sep 15;23(1):780. doi: 10.1186/s13063-022-06634-w. Trials. 2022. PMID: 36109788 Free PMC article.
-
Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study.Br J Nutr. 2018 Jan;119(2):176-189. doi: 10.1017/S0007114517003440. Epub 2018 Jan 8. Br J Nutr. 2018. PMID: 29307330 Clinical Trial.
-
The effects of inulin on gut microbial composition: a systematic review of evidence from human studies.Eur J Clin Microbiol Infect Dis. 2020 Mar;39(3):403-413. doi: 10.1007/s10096-019-03721-w. Epub 2019 Nov 9. Eur J Clin Microbiol Infect Dis. 2020. PMID: 31707507
-
The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Nutrients. 2020 Oct 23;12(11):3239. doi: 10.3390/nu12113239. Nutrients. 2020. PMID: 33113929 Free PMC article.
Cited by
-
Characterization of gut microbiota and metabolites in renal transplant recipients during COVID-19 and prediction of one-year allograft function.J Transl Med. 2025 Apr 10;23(1):420. doi: 10.1186/s12967-025-06090-5. J Transl Med. 2025. PMID: 40211390 Free PMC article.
-
Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review.Int J Mol Sci. 2025 Jan 20;26(2):862. doi: 10.3390/ijms26020862. Int J Mol Sci. 2025. PMID: 39859575 Free PMC article. Review.
References
-
- Elias M., Pievani D., Randoux C., Louis K., Denis B., Delion A., Le Goff O., Antoine C., Greze C., Pillebout E. COVID-19 infection in kidney transplant recipients: Disease incidence and clinical outcomes. J. Am. Soc. Nephrol. JASN. 2020;31:2413–2423. doi: 10.1681/ASN.2020050639. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

