Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024)
- PMID: 38932369
- PMCID: PMC11209424
- DOI: 10.3390/vaccines12060640
Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024)
Abstract
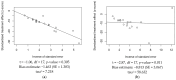
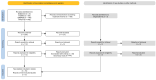
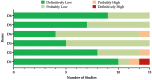
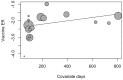
A systematic review with a meta-analysis was performed to gather available evidence on the effectiveness of monoclonal antibody nirsevimab in the prevention of lower respiratory tract diseases (LRTDs) due to respiratory syncytial virus (RSV) in children and newborns (CRD42024540669). Studies reporting on real-world experience and randomized controlled trials (RCTs) were searched for in three databases (PubMed, Embase, and Scopus) until 1 May 2024. Our analysis included five RCTs, seven real-world reports, and one official report from the health authorities. Due to the cross-reporting of RCTs and the inclusion of multiple series in a single study, the meta-analysis was performed on 45,238 infants from 19 series. The meta-analysis documented a pooled immunization efficacy of 88.40% (95% confidence interval (95% CI) from 84.70 to 91.21) on the occurrence of hospital admission due to RSV, with moderate heterogeneity (I2 24.3%, 95% CI 0.0 to 56.6). Immunization efficacy decreased with the overall length of the observation time (Spearman's r = -0.546, p = 0.016), and the risk of breakthrough infections was substantially greater in studies with observation times ≥150 days compared to studies lasting <150 days (risk ratio 2.170, 95% CI 1.860 to 2.532). However, the effect of observation time in meta-regression analysis was conflicting (β = 0.001, 95% CI -0.001 to 0.002; p = 0.092). In conclusion, the delivery of nirsevimab was quite effective in preventing hospital admissions due to LRTDs. However, further analyses of the whole RSV season are required before tailoring specific public health interventions.
Keywords: RSV; immunization; monoclonal antibodies; viral pneumonia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- Shi T., Denouel A., Tietjen A.K., Campbell I., Moran E., Li X., Campbell H., Demont C., Nyawanda B.O., Chu H.Y., et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021;222:S577–S583. doi: 10.1093/INFDIS/JIZ059. - DOI - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/s0140-6736(22)00478-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

