Molecular Events in Immune Responses to Sublingual Influenza Vaccine with Hemagglutinin Antigen and Poly(I:C) Adjuvant in Nonhuman Primates, Cynomolgus Macaques
- PMID: 38932372
- PMCID: PMC11209156
- DOI: 10.3390/vaccines12060643
Molecular Events in Immune Responses to Sublingual Influenza Vaccine with Hemagglutinin Antigen and Poly(I:C) Adjuvant in Nonhuman Primates, Cynomolgus Macaques
Abstract
Sublingual vaccines offer the benefits of inducing mucosal immunity to protect against respiratory viruses, including Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and influenza, while also enabling needle-free self-administration. In a previous study, a sublingual SARS-CoV-2 vaccination was created by combining a recombinafigureCoV-2 spike protein receptor-binding domain antigen with a double strand RNA Poly(I:C) adjuvant. This vaccine was tested on nonhuman primates, Cynomolgus macaques. This study examined the immune and inflammatory responses elicited by the sublingual influenza vaccine containing hemagglutinin (HA) antigen and Poly(I:C) adjuvants, and assessed the safety of this vaccine in nonhuman primates. The Poly(I:C)-adjuvanted sublingual vaccine induced both mucosal and systemic immunities. Specifically, the sublingual vaccine produced HA-specific secretory IgA antibodies in saliva and nasal washings, and HA-specific IgA and IgG were detected in the blood. This vaccine appeared to be safe, as judged from the results of blood tests and plasma C-reactive protein levels. Notably, sublingual vaccination neither increased the production of inflammation-associated cytokines-IFN-alpha, IFN-gamma, and IL-17-in the blood, nor upregulated the gene expression of proinflammatory cytokines-IL12A, IL12B, IFNA1, IFNB1, CD69, and granzyme B-in white blood cells. Moreover, DNA microarray analyses revealed that sublingual vaccination evoked both enhancing and suppressing expression changes in genes associated with immune-related responses in cynomolgus monkeys. Therefore, the sublingual vaccine with the Poly(I:C) adjuvant is safe, and creates a balanced state of enhancing and suppressing the immune-related response.
Keywords: CLEC4G; LSECtin; efficacy; monkey; prophylaxis; tolerance.
Conflict of interest statement
All authors have completed the DPCOI form and declare that: (i) no support, financial or otherwise, has been received from any organization that may have an interest in the submitted manuscript; and (ii) there are no other relationships or activities that could appear to have influenced the submitted manuscript.
Figures
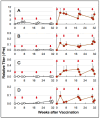
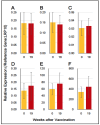
 ) indicate the control group, and solid brown circles (
) indicate the control group, and solid brown circles ( ) indicate the experiment/HA + Poly(I:C)) group. Red arrows (
) indicate the experiment/HA + Poly(I:C)) group. Red arrows ( ) indicate vaccination. Relative titers were estimated using the conditions mentioned in Section 2.
) indicate vaccination. Relative titers were estimated using the conditions mentioned in Section 2.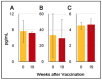
Similar articles
-
SARS-CoV-2 sublingual vaccine with RBD antigen and poly(I:C) adjuvant: Preclinical study in cynomolgus macaques.Biol Methods Protoc. 2023 Sep 13;8(1):bpad017. doi: 10.1093/biomethods/bpad017. eCollection 2023. Biol Methods Protoc. 2023. PMID: 37711440 Free PMC article.
-
Intranasal influenza vaccination using a new synthetic mucosal adjuvant SF-10: induction of potent local and systemic immunity with balanced Th1 and Th2 responses.Influenza Other Respir Viruses. 2013 Nov;7(6):1218-26. doi: 10.1111/irv.12124. Epub 2013 May 26. Influenza Other Respir Viruses. 2013. PMID: 23710832 Free PMC article.
-
A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung.Vaccine. 2014 May 30;32(26):3216-22. doi: 10.1016/j.vaccine.2014.04.011. Epub 2014 Apr 13. Vaccine. 2014. PMID: 24731807
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
Safety Assessment of a Sublingual Vaccine Formulated with Poly(I:C) Adjuvant and Influenza HA Antigen in Mice and Macaque Monkeys: Comparison with Intranasal Vaccine.Vaccines (Basel). 2025 Feb 28;13(3):261. doi: 10.3390/vaccines13030261. Vaccines (Basel). 2025. PMID: 40266106 Free PMC article.
-
Advances in protein subunit vaccines against H1N1/09 influenza.Front Immunol. 2024 Nov 22;15:1499754. doi: 10.3389/fimmu.2024.1499754. eCollection 2024. Front Immunol. 2024. PMID: 39650643 Free PMC article. Review.
-
Intranasal Immunization with DNA Vaccine HA-CCL19/Polyethylenimine/Chitosan Composite Provides Immune Protection Against H7N9 Infection.Vaccines (Basel). 2024 Dec 26;13(1):10. doi: 10.3390/vaccines13010010. Vaccines (Basel). 2024. PMID: 39852789 Free PMC article.
References
-
- World Health Organization Influenza (Seasonal) 2023. [(accessed on 9 April 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- World Health Organization Vaccines Against Influenza: WHO Position Paper—May 2022. [(accessed on 9 April 2024)]. Available online: https://www.who.int/publications/i/item/who-wer9719.
-
- Lemiale F., Kong W., Akyurek L.M., Ling X., Huang Y., Chakrabarti B.K., Eckhaus M., Nabel G.J. Enhanced mucosal immunoglobulin a response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 2003;77:10078–10087. doi: 10.1128/jvi.77.18.10078-10087.2003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

