SARS-CoV-2 Humoral and Cellular Immune Responses in People Living with HIV
- PMID: 38932392
- PMCID: PMC11209143
- DOI: 10.3390/vaccines12060663
SARS-CoV-2 Humoral and Cellular Immune Responses in People Living with HIV
Abstract
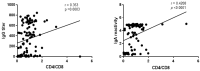
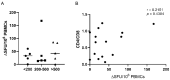
Immunosuppressed individuals, such as people living with HIV (PLWH), remain vulnerable to severe COVID-19. We analyzed the persistence of specific SARS-CoV-2 humoral and cellular immune responses in a retrospective, cross-sectional study in PLWH on antiretroviral therapy. Among 104 participants, 70.2% had anti-S IgG antibodies, and 55.8% had significant neutralizing activity against the Omicron variant in a surrogate virus neutralization test. Only 38.5% were vaccinated (8.76 ± 4.1 months prior), all displaying anti-S IgG, 75% with neutralizing antibodies and anti-S IgA. Overall, 29.8% of PLWH had no SARS-CoV-2 serologic markers; they displayed significantly lower CD4 counts and higher HIV viral load. Severe immunosuppression (present in 12.5% of participants) was linked to lower levels of detectable anti-S IgG (p = 0.0003), anti-S IgA (p < 0.0001) and lack of neutralizing activity against the Omicron variant (p < 0.0001). T-cell responses were present in 86.7% of tested participants, even in those lacking serological markers. In PLWH without severe immunosuppression, neutralizing antibodies and T-cell responses persisted for up to 9 months post-infection or vaccination. Advanced immunosuppression led to diminished humoral immune responses but retained specific cellular immunity.
Keywords: COVID-19; SARS-CoV-2; cellular; humoral immune response; immunosuppression; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers.Vaccines (Basel). 2022 Dec 13;10(12):2129. doi: 10.3390/vaccines10122129. Vaccines (Basel). 2022. PMID: 36560539 Free PMC article.
-
Cluster Analysis Identifies Distinct Patterns of T-Cell and Humoral Immune Responses Evolution Following a Third Dose of SARS-CoV-2 Vaccine in People Living with HIV.Viruses. 2023 Jun 26;15(7):1435. doi: 10.3390/v15071435. Viruses. 2023. PMID: 37515123 Free PMC article.
-
Trends of humoral immune responses to heterologous antigenic exposure due to vaccination & omicron SARS-CoV-2 infection: Implications for boosting.Indian J Med Res. 2023 Jun;157(6):509-518. doi: 10.4103/ijmr.ijmr_2521_22. Indian J Med Res. 2023. PMID: 37322634 Free PMC article.
-
Humoral immune response in people living with HIV after administration of SARS-CoV-2 vaccine CoronaVac or BNT162b2 or CoronaVac/BNT162b2 booster sequence: A cross-sectional study.Vaccine. 2024 Jan 25;42(3):671-676. doi: 10.1016/j.vaccine.2023.12.035. Epub 2023 Dec 20. Vaccine. 2024. PMID: 38123398
-
SARS-CoV-2 immunity and vaccine strategies in people with HIV.Oxf Open Immunol. 2022 Aug 17;3(1):iqac005. doi: 10.1093/oxfimm/iqac005. eCollection 2022. Oxf Open Immunol. 2022. PMID: 36846557 Free PMC article. Review.
References
-
- Bertagnolio S., Thwin S.S., Silva R., Nagarajan S., Jassat W., Fowler R., Haniffa R., Reveiz L., Ford N., Doherty M., et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: Analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. 2022;9:e486–e495. doi: 10.1016/S2352-3018(22)00097-2. - DOI - PMC - PubMed
-
- European AIDS . Clinical Society EACS Guidelines, Version 12.0, Oct 2023 PART IV. European AIDS; Brussels, Belgium: 2023. [(accessed on 2 March 2024)]. p. 151. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf.
-
- World Health Organization SAGE Updates COVID-19 Vaccination Guidance. [(accessed on 22 February 2024)]. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immuni....
Grants and funding
- Contract no. 33PFE/30.12.2021, funded by the Ministry of Research and Innovation within PNCDI III, Program 1-Development of the National RD system, Subprogram 1.2-Institutional Performance-RDI excellence funding projects/Carol Davila University of Medicine and Pharmacy
- CNCS-UEFISCDI, project number PN-III-P1-1.1-PD-2021-0825, within PNCDI III/Ministry of Research, Innovation and Digitization
- through the institutional program Publish not Perish/Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

