Lipid Nanoparticles Outperform Electroporation in Delivering Therapeutic HPV DNA Vaccines
- PMID: 38932395
- PMCID: PMC11209142
- DOI: 10.3390/vaccines12060666
Lipid Nanoparticles Outperform Electroporation in Delivering Therapeutic HPV DNA Vaccines
Abstract
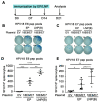
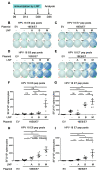
Therapeutic HPV vaccines that induce potent HPV-specific cellular immunity and eliminate pre-existing infections remain elusive. Among various candidates under development, those based on DNA constructs are considered promising because of their safety profile, stability, and efficacy. However, the use of electroporation (EP) as a main delivery method for such vaccines is notorious for adverse effects like pain and potentially irreversible muscle damage. Moreover, the requirement for specialized equipment adds to the complexity and cost of clinical applications. As an alternative to EP, lipid nanoparticles (LNPs) that are already commercially available for delivering mRNA and siRNA vaccines are likely to be feasible. Here, we have compared three intramuscular delivery systems in a preclinical setting. In terms of HPV-specific cellular immune responses, mice receiving therapeutic HPV DNA vaccines encapsulated with LNP demonstrated superior outcomes when compared to EP administration, while the naked plasmid vaccine showed negligible responses, as expected. In addition, SM-102 LNP M exhibited the most promising results in delivering candidate DNA vaccines. Thus, LNP proves to be a feasible delivery method in vivo, offering improved immunogenicity over traditional approaches.
Keywords: DNA vaccine; cell-mediated immunity; electroporation (EP); lipid nanoparticles (LNPs); therapeutic HPV vaccine.
Conflict of interest statement
X.L., L.L., X.Z. (Xiaopeng Zhang), P.H., X.W. (Xiaoyu Wu), S.J., X.W. (Xingxing Wang) and X.Z. (Xiujun Zhang) are employees of Aeonvital Biomedical Research Institute, with X.Z. holding stakes in this private company.
Figures


Similar articles
-
Lipid nanoparticles deliver DNA-encoded biologics and induce potent protective immunity.Mol Cancer. 2025 Jan 13;24(1):12. doi: 10.1186/s12943-024-02211-8. Mol Cancer. 2025. PMID: 39806486 Free PMC article.
-
The Expression Kinetics and Immunogenicity of Lipid Nanoparticles Delivering Plasmid DNA and mRNA in Mice.Vaccines (Basel). 2023 Oct 11;11(10):1580. doi: 10.3390/vaccines11101580. Vaccines (Basel). 2023. PMID: 37896985 Free PMC article.
-
Electroporation of a nanoparticle-associated DNA vaccine induces higher inflammation and immunity compared to its delivery with microneedle patches in pigs.J Control Release. 2019 Aug 28;308:14-28. doi: 10.1016/j.jconrel.2019.06.041. Epub 2019 Jun 29. J Control Release. 2019. PMID: 31265882
-
Chemistry of Lipid Nanoparticles for RNA Delivery.Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review.
-
The role of lipid components in lipid nanoparticles for vaccines and gene therapy.Adv Drug Deliv Rev. 2022 Sep;188:114416. doi: 10.1016/j.addr.2022.114416. Epub 2022 Jul 3. Adv Drug Deliv Rev. 2022. PMID: 35787388 Free PMC article. Review.
Cited by
-
Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines.Vaccines (Basel). 2025 Jan 19;13(1):92. doi: 10.3390/vaccines13010092. Vaccines (Basel). 2025. PMID: 39852871 Free PMC article. Review.
-
Lipid nanoparticles deliver DNA-encoded biologics and induce potent protective immunity.Mol Cancer. 2025 Jan 13;24(1):12. doi: 10.1186/s12943-024-02211-8. Mol Cancer. 2025. PMID: 39806486 Free PMC article.
-
Vaccine-Based Immunotherapy for Oropharyngeal and Nasopharyngeal Cancers.J Clin Med. 2025 Feb 11;14(4):1170. doi: 10.3390/jcm14041170. J Clin Med. 2025. PMID: 40004705 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Bsc M.F.B., Me J.F., Soerjomataram M.I., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

