S2 Peptide-Conjugated SARS-CoV-2 Virus-like Particles Provide Broad Protection against SARS-CoV-2 Variants of Concern
- PMID: 38932406
- PMCID: PMC11209314
- DOI: 10.3390/vaccines12060676
S2 Peptide-Conjugated SARS-CoV-2 Virus-like Particles Provide Broad Protection against SARS-CoV-2 Variants of Concern
Abstract
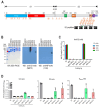
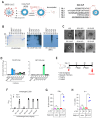
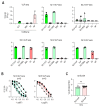
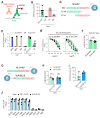
Approved COVID-19 vaccines primarily induce neutralizing antibodies targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein. However, the emergence of variants of concern with RBD mutations poses challenges to vaccine efficacy. This study aimed to design a next-generation vaccine that provides broader protection against diverse coronaviruses, focusing on glycan-free S2 peptides as vaccine candidates to overcome the low immunogenicity of the S2 domain due to the N-linked glycans on the S antigen stalk, which can mask S2 antibody responses. Glycan-free S2 peptides were synthesized and attached to SARS-CoV-2 virus-like particles (VLPs) lacking the S antigen. Humoral and cellular immune responses were analyzed after the second booster immunization in BALB/c mice. Enzyme-linked immunosorbent assay revealed the reactivity of sera against SARS-CoV-2 variants, and pseudovirus neutralization assay confirmed neutralizing activities. Among the S2 peptide-conjugated VLPs, the S2.3 (N1135-K1157) and S2.5 (A1174-L1193) peptide-VLP conjugates effectively induced S2-specific serum immunoglobulins. These antisera showed high reactivity against SARS-CoV-2 variant S proteins and effectively inhibited pseudoviral infections. S2 peptide-conjugated VLPs activated SARS-CoV-2 VLP-specific T-cells. The SARS-CoV-2 vaccine incorporating conserved S2 peptides and CoV-2 VLPs shows promise as a universal vaccine capable of generating neutralizing antibodies and T-cell responses against SARS-CoV-2 variants.
Keywords: SARS-CoV-2; broadly neutralizing antibody; conserved S2; peptide conjugation; universal vaccine; virus-like particles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Anamnestic humoral correlates of immunity across SARS-CoV-2 variants of concern.mBio. 2023 Aug 31;14(4):e0090223. doi: 10.1128/mbio.00902-23. Epub 2023 Aug 3. mBio. 2023. PMID: 37535402 Free PMC article.
-
Neutralizing Monoclonal Antibodies Inhibit SARS-CoV-2 Infection through Blocking Membrane Fusion.Microbiol Spectr. 2022 Apr 27;10(2):e0181421. doi: 10.1128/spectrum.01814-21. Epub 2022 Mar 16. Microbiol Spectr. 2022. PMID: 35293796 Free PMC article.
-
Novel S2 subunit-specific antibody with broad neutralizing activity against SARS-CoV-2 variants of concern.Front Immunol. 2023 Dec 8;14:1307693. doi: 10.3389/fimmu.2023.1307693. eCollection 2023. Front Immunol. 2023. PMID: 38143750 Free PMC article.
-
SARS-CoV-2 spike S2-specific neutralizing antibodies.Emerg Microbes Infect. 2023 Dec;12(2):2220582. doi: 10.1080/22221751.2023.2220582. Emerg Microbes Infect. 2023. PMID: 37254830 Free PMC article. Review.
-
VLP-Based COVID-19 Vaccines: An Adaptable Technology against the Threat of New Variants.Vaccines (Basel). 2021 Nov 30;9(12):1409. doi: 10.3390/vaccines9121409. Vaccines (Basel). 2021. PMID: 34960155 Free PMC article. Review.
Cited by
-
Rerouting therapeutic peptides and unlocking their potential against SARS-CoV2.3 Biotech. 2025 May;15(5):116. doi: 10.1007/s13205-025-04270-0. Epub 2025 Apr 4. 3 Biotech. 2025. PMID: 40191455 Review.
References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

