A methodological quality review of citations of randomized controlled trials of diabetes type2 in leading clinical practice guidelines and systematic reviews
- PMID: 38932844
- PMCID: PMC11196434
- DOI: 10.1007/s40200-023-01328-9
A methodological quality review of citations of randomized controlled trials of diabetes type2 in leading clinical practice guidelines and systematic reviews
Abstract
Objective: Evaluate methodological quality of type 2 diabetes RCTs conducted in Iran and cited in clinical practice guidelines and systematic reviews and meta-analyses.
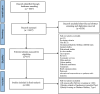
Methods: We conducted a descriptive methodological quality review, analyzing 286 Randomized Controlled Trials (RCTs) on diabetes mellitus published in Iran from July 2004 to 2021. We searched six databases systematically and evaluated eligible articles using the CONSORT 2010 checklist for abstracts. Two investigators assessed the data using a 17-item checklist derived from CONSORT. Additionally, we examined the citations of each RCT in 260 clinical practice guidelines, with a specific focus on the adequate reporting of outcomes.
Results: Out of 6667 articles, 286 analyzed. Poor reporting and failure to meet criteria observed. Only 3.8% cited in guidelines. Reporting rates: primary outcomes (41.9%), randomization (61.8%), trial recruitment (12.6%), blinding (50.8%). 27.9% cited in systematic reviews, 50.34% in systematic reviews and meta-analyses, 26.57% in meta-analyses. 67.8% of papers cited in systematic reviews. Adherence highest for participants, objective, randomization, intervention, outcome; lowest for recruitment, trial design, funding source, harms, and reporting primary outcomes.
Conclusions: Poor methodological reporting and adherence to CONSORT checklist in evaluated RCTs, especially in methodological sections. Improvements needed for reliable and applicable results in guidelines, reviews, and meta-analyses. Inadequate outcome reporting challenges researchers, clinicians, and policymakers, impacting evidence-based decision-making. Urgent improvements in RCT registration necessary.
Keywords: Clinical practice guideline; Diabetes; Randomized controlled trials; Reporting.
© The Author(s), under exclusive licence to Tehran University of Medical Sciences 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interests that are relevant to the content of this article.
References
-
- Data from: Global report on diabetes [Database on the internet] WHO deposited.2016
-
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. - PubMed
-
- Kononenko IV, Smirnova OM, Mayorov AY, Shestakova MV. Classification of diabetes. World Health Organization 2019. What’s new? Diabetes Mellitus. 2020;23(4):329–39.
-
- Organization WH. Addressing diabetes as a public health challenge in the Eastern Mediterranean Region. World Health Organization.Regional Office for the Eastern Mediterranean. 2021.
Publication types
LinkOut - more resources
Full Text Sources