The effects of meal patterns on liver steatosis, fibrosis, and biochemical factors in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial
- PMID: 38932893
- PMCID: PMC11196559
- DOI: 10.1007/s40200-023-01375-2
The effects of meal patterns on liver steatosis, fibrosis, and biochemical factors in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial
Abstract
Background: This study was designed to compare the effects of four meal patterns on liver steatosis, fibrosis, and biochemical factors in patients with Nonalcoholic fatty liver disease (NAFLD).
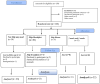
Methods: The 12-week intervention was performed on 123 patients with NAFLD who were randomly allocated into four groups: "3-meals", "skipping breakfast", "skipping dinner", and " 3 meals and 3 snacks per day." group. The assessment of liver steatosis, fibrosis, biochemical factors, and anthropometrical evaluation were performed at baseline and at end of the study.
Results: A significant improvement was found in the liver steatosis and fibrosis among the patients who consumed 3 meals, 3 snacks compared to the other groups (P < 0.001). In addition, a higher reduction was observed in serum levels of alanine amino transferase (ALT) (20.93 ± 23.37 mg/dl, P < 0.001), aspartate aminotransferase (AST) (17.15 ± 16.48 mg/dl, P < 0.001), gamma-glutamyl transferase(GGT) (13.43 ± 13.41 mg/dl; P < 0.001), and alkaline phosphatase (ALK) (47.19 ± 60.51 mg/dl; P = 0.004) in patients who consumed 3 meals, 3 snacks, while the concentration of liver enzymes in patients who consumed 3 meals increased significantly. At the end of the study, there was a significant increase in the fasting blood sugar (FBS) concentration in the "skipping breakfast" group (17.51 ± 38.85 mg/dl; P = 0.011) and "3-meals" group (17.51 ± 38.85 mg/dl, P = 0.03).
Conclusion: Consuming 3 meals, 3 snack per day significantly improves disease severity and biochemical factors in NAFLD patients. Further studies are warranted.
Trial registration number: : IRCT20201010048982N2. Name of the registry: Urmia University of Medical Sciences. Date of registration: 2021-08-22, 1400/05/31. URL of trial registry record: https://www.irct.ir/search/result?query=IRCT20201010048982N2.
Supplementary information: The online version contains supplementary material available at 10.1007/s40200-023-01375-2.
Keywords: Clinical trial; Liver enzymes; Meal pattern; Non-alcoholic fatty Liver Disease; Steatosis.
© The Author(s), under exclusive licence to Tehran University of Medical Sciences 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Similar articles
-
Dietary approaches to stop hypertension (DASH) diet improves hepatic fibrosis, steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: a randomized controlled trial.Eur J Nutr. 2024 Feb;63(1):95-105. doi: 10.1007/s00394-023-03221-w. Epub 2023 Oct 19. Eur J Nutr. 2024. PMID: 37855891 Clinical Trial.
-
The effect of a fruit-rich diet on liver biomarkers, insulin resistance, and lipid profile in patients with non-alcoholic fatty liver disease: a randomized clinical trial.Scand J Gastroenterol. 2022 Oct;57(10):1238-1249. doi: 10.1080/00365521.2022.2071109. Epub 2022 Jun 16. Scand J Gastroenterol. 2022. PMID: 35710164 Clinical Trial.
-
The effect of daily consumption of probiotic yogurt on liver enzymes, steatosis and fibrosis in patients with nonalcoholic fatty liver disease (NAFLD): study protocol for a randomized clinical trial.BMC Gastroenterol. 2022 Mar 7;22(1):102. doi: 10.1186/s12876-022-02176-2. BMC Gastroenterol. 2022. PMID: 35255811 Free PMC article.
-
Vitamin E supplementation in the treatment on nonalcoholic fatty liver disease (NAFLD): Evidence from an umbrella review of meta-analysis on randomized controlled trials.J Dig Dis. 2023 Jun-Jul;24(6-7):380-389. doi: 10.1111/1751-2980.13210. Epub 2023 Sep 2. J Dig Dis. 2023. PMID: 37503812
-
The effects of probiotic supplementation and exercise training on liver enzymes and cardiometabolic markers in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized clinical trials.Nutr Metab (Lond). 2024 Aug 1;21(1):59. doi: 10.1186/s12986-024-00826-8. Nutr Metab (Lond). 2024. PMID: 39090657 Free PMC article. Review.
Cited by
-
The role of irregular eating behaviors in metabolic dysfunction-associated steatotic liver disease: evidence from a multicenter cross-sectional study in China.J Transl Med. 2025 Aug 2;23(1):859. doi: 10.1186/s12967-025-06870-z. J Transl Med. 2025. PMID: 40753232 Free PMC article.
References
-
- Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recen Clin Trial. 2014;9(3):126–33. - PubMed
-
- Bellentani S. The epidemiology of non-alcoholic fatty Liver Disease. Liver Int. 2017;37(Suppl 1):81–4. - PubMed
-
- Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Reviews Gastroenterol Hepatol. 2016;13(4):196–205. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous