Utilization of Ningxiang pig milk oligosaccharides by Akkermansia muciniphila in vitro fermentation: enhancing neonatal piglet survival
- PMID: 38933035
- PMCID: PMC11199860
- DOI: 10.3389/fmicb.2024.1430276
Utilization of Ningxiang pig milk oligosaccharides by Akkermansia muciniphila in vitro fermentation: enhancing neonatal piglet survival
Abstract
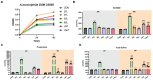
Akkermansia muciniphila (A. muciniphila), an intestinal symbiont residing in the mucosal layer, shows promise as a probiotic. Our previous study found that the abundance of A. muciniphila was significantly higher in Ningxiang suckling piglets compared to other breeds, suggesting that early breast milk may play a crucial role. This study examines A. muciniphila's ability to utilize Ningxiang pig milk oligosaccharides. We discovered that A. muciniphila can thrive on both Ningxiang pig colostrum and purified pig milk oligosaccharides. Genetic analysis has shown that A. muciniphila harbors essential glycan-degrading enzymes, enabling it to effectively break down a broad spectrum of oligosaccharides. Our findings demonstrate that A. muciniphila can degrade pig milk oligosaccharides structures such as 3'-FL, 3'-SL, LNT, and LNnT, producing short-chain fatty acids in the process. The hydrolysis of these host-derived glycan structures enhances A. muciniphila's symbiotic interactions with other beneficial gut bacteria, contributing to a dynamic microbial ecological network. The capability of A. muciniphila to utilize pig milk oligosaccharides allows it to establish itself in the intestines of newborn piglets, effectively colonizing the mucosal layer early in life. This early colonization is key in supporting both mucosal and metabolic health, which is critical for enhancing piglet survival during lactation.
Keywords: Akkermansia muciniphila; Ningxiang pig; milk oligosaccharides; piglet survival; short-chain fatty acids.
Copyright © 2024 Zhang, Wu, Kang, Wang and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Barile D., Marotta M., Chu C., Mehra R., Grimm R., Lebrilla C. B., et al. . (2010). Neutral and acidic oligosaccharides in Holstein-Friesian colostrum during the first 3 days of lactation measured by high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. J. Dairy Sci. 93, 3940–3949. doi: 10.3168/jds.2010-3156, PMID: - DOI - PMC - PubMed
-
- Bridgman S. L., Azad M. B., Field C. J., Haqq A. M., Becker A. B., Mandhane P. J., et al. . (2017). Fecal short-chain fatty acid variations by breastfeeding status in infants at 4 months: differences in relative versus absolute concentrations. Front. Nutr. 4:11. doi: 10.3389/fnut.2017.00011, PMID: - DOI - PMC - PubMed
-
- Collado M. C., Derrien M., Isolauri E., de Vos W. M., Salminen S. (2007). Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 73, 7767–7770. doi: 10.1128/AEM.01477-07, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

