Dendritic morphology of motor neurons and interneurons within the compact, semicompact, and loose formations of the rat nucleus ambiguus
- PMID: 38933178
- PMCID: PMC11199410
- DOI: 10.3389/fncel.2024.1409974
Dendritic morphology of motor neurons and interneurons within the compact, semicompact, and loose formations of the rat nucleus ambiguus
Abstract
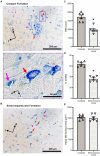
Introduction: Motor neurons (MNs) within the nucleus ambiguus innervate the skeletal muscles of the larynx, pharynx, and oesophagus. These muscles are activated during vocalisation and swallowing and must be coordinated with several respiratory and other behaviours. Despite many studies evaluating the projections and orientation of MNs within the nucleus ambiguus, there is no quantitative information regarding the dendritic arbours of MNs residing in the compact, and semicompact/loose formations of the nucleus ambiguus..
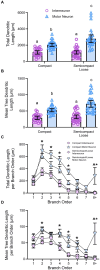
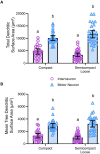
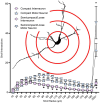
Methods: In female and male Fischer 344 rats, we evaluated MN number using Nissl staining, and MN and non-MN dendritic morphology using Golgi-Cox impregnation Brightfield imaging of transverse Nissl sections (15 μm) were taken to stereologically assess the number of nucleus ambiguus MNs within the compact and semicompact/loose formations. Pseudo-confocal imaging of Golgi-impregnated neurons within the nucleus ambiguus (sectioned transversely at 180 μm) was traced in 3D to determine dendritic arbourisation.
Results: We found a greater abundance of MNs within the compact than the semicompact/loose formations. Dendritic lengths, complexity, and convex hull surface areas were greatest in MNs of the semicompact/loose formation, with compact formation MNs being smaller. MNs from both regions were larger than non-MNs reconstructed within the nucleus ambiguus.
Conclusion: Adding HBLS to the diet could be a potentially effective strategy to improve horses' health.
Keywords: Golgi–Cox; brainstem; convex hull; swallow; vocalisation.
Copyright © 2024 Fogarty.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dendritic alterations precede age-related dysphagia and nucleus ambiguus motor neuron death.J Physiol. 2025 Mar;603(5):1299-1321. doi: 10.1113/JP287457. Epub 2025 Jan 27. J Physiol. 2025. PMID: 39868939 Free PMC article.
-
Ultrastructure and synaptology of the nucleus ambiguus in the rat: the semicompact and loose formations.J Comp Neurol. 1996 Nov 4;375(1):109-27. doi: 10.1002/(SICI)1096-9861(19961104)375:1<109::AID-CNE7>3.0.CO;2-7. J Comp Neurol. 1996. PMID: 8913896
-
Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus.J Comp Neurol. 1987 Aug 22;262(4):546-62. doi: 10.1002/cne.902620408. J Comp Neurol. 1987. PMID: 3667964
-
Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing.Am J Med. 2000 Mar 6;108 Suppl 4a:79S-86S. doi: 10.1016/s0002-9343(99)00343-5. Am J Med. 2000. PMID: 10718457 Review.
-
Central integration of swallow and airway-protective reflexes.Am J Med. 2000 Mar 6;108 Suppl 4a:62S-67S. doi: 10.1016/s0002-9343(99)00340-x. Am J Med. 2000. PMID: 10718454 Review.
Cited by
-
Timeline of hypoglossal motor neuron death and intrinsic tongue muscle denervation in high-copy number SOD1G93A mice.Front Neurol. 2024 Jul 25;15:1422943. doi: 10.3389/fneur.2024.1422943. eCollection 2024. Front Neurol. 2024. PMID: 39119557 Free PMC article.
-
Dendritic alterations precede age-related dysphagia and nucleus ambiguus motor neuron death.J Physiol. 2025 Mar;603(5):1299-1321. doi: 10.1113/JP287457. Epub 2025 Jan 27. J Physiol. 2025. PMID: 39868939 Free PMC article.
-
Molecular and functional diversity of the autonomic nervous system.Nat Rev Neurosci. 2025 Jul 3. doi: 10.1038/s41583-025-00941-2. Online ahead of print. Nat Rev Neurosci. 2025. PMID: 40610604 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

