Olea europaea leaf exosome-like nanovesicles encapsulated in a hyaluronic acid / tannic acid hydrogel dressing with dual "defense-repair" effects for treating skin photoaging
- PMID: 38933415
- PMCID: PMC11201150
- DOI: 10.1016/j.mtbio.2024.101103
Olea europaea leaf exosome-like nanovesicles encapsulated in a hyaluronic acid / tannic acid hydrogel dressing with dual "defense-repair" effects for treating skin photoaging
Abstract
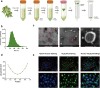
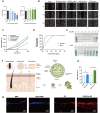
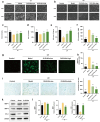
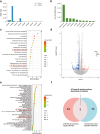
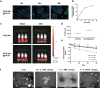
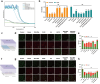
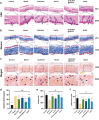
Photoaging, primarily caused by ultraviolet (UV) light, is the major factor in extrinsic skin aging. Existing anti-photoaging strategies mainly focus on early sun protection or repairing damaged skin, lacking a comprehensive treatment strategy. Therefore, this study developed a dressing that actively shields against UV radiation and repairs photoaged skin, offering double protection. This study utilized exosome-like nanovesicles derived from Olea europaea leaves (OLELNVs), enhancing them into a potent core biomaterial with high-dose effects and skin-friendly, non-cytotoxic inhibition of cell aging. These nanovesicles were incorporated into a cross-linked hyaluronic acid (HA) and tannic acid (TA) hydrogel with strong UV-absorbing properties, creating the OLELNVs@HA/TA hydrogel system. In vitro and in vivo experiments demonstrated that OLELNVs@HA/TA hydrogel can effectively reduce UV-induced skin damage and promote skin repair and regeneration. Additionally, RNA-seq and clustering analysis of miR168a-5p predicted targets revealed significant down-regulation of the NF-κB signaling pathway, mediating inflammatory aging responses. Overall, the OLELNVs@HA/TA hydrogel represents a novel dual-strategy approach for clinical application in combating photoaging.
Keywords: Exosome-like nanovesicles; Extracellular vesicles; Hyaluronic acid hydrogel; Photoaging; Skin regeneration; UV shielding.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Kligman L.H., Kligman A.M. The nature of photoaging: its prevention and repair. PubMed. 1986;3:215–227. https://pubmed.ncbi.nlm.nih.gov/3534809 - PubMed
LinkOut - more resources
Full Text Sources

