Blockade of the CD47/SIRPα checkpoint axis potentiates the macrophage-mediated antitumor efficacy of tafasitamab
- PMID: 38934068
- PMCID: PMC11609795
- DOI: 10.3324/haematol.2023.284795
Blockade of the CD47/SIRPα checkpoint axis potentiates the macrophage-mediated antitumor efficacy of tafasitamab
Abstract
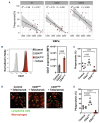
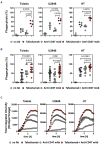
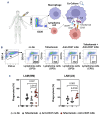
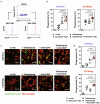
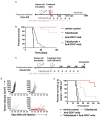
Macrophages are one of the key mediators of the therapeutic effects exerted by monoclonal antibodies, such as the anti-CD19 antibody tafasitamab, approved in combination with lenalidomide for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL). However, antibody-dependent cellular phagocytosis (ADCP) in the tumor microenvironment can be counteracted by increased expression of the inhibitory receptor SIRPα on macrophages and its ligand, the immune checkpoint molecule CD47, on tumor cells. The aim of this study was to investigate the impact of the CD47-SIRPα axis on tafasitamab- mediated phagocytosis and explore the potential of anti-CD47 blockade to enhance its antitumor activity. Elevated expression of both SIRPα and CD47 was observed in DLBCL patient-derived lymph node biopsies compared to healthy control lymph nodes. CRISPR-mediated CD47 overexpression affected tafasitamab-mediated ADCP in vitro and increased expression of SIRPα on macrophages correlated with decreased ADCP activity of tafasitamab against DLBCL cell lines. A combination of tafasitamab and an anti-CD47 blocking antibody enhanced ADCP activity of in vitro-generated macrophages. Importantly, tafasitamab-mediated phagocytosis was elevated in combination with CD47 blockade using primary DLBCL cells and patient-derived lymphoma-associated macrophages in an autologous setting. Furthermore, lymphoma cells with low CD19 expression were efficiently eliminated by the combination treatment. Finally, combined treatment of tafasitamab and an anti-CD47 antibody resulted in enhanced tumor volume reduction and survival benefit in lymphoma xenograft mouse models. These findings provide evidence that CD47 blockade can enhance the phagocytic potential of tumor-targeting immunotherapies such as tafasitamab and suggest that there is value in exploring the combination in the clinic.
Figures

References
-
- Pfreundschuh M, Kuhnt E, Trumper L, et al. . CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013-1022. - PubMed
-
- Horton HM, Bernett MJ, Pong E, et al. . Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049-8057. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

