Adverse event comparison between glucagon-like peptide-1 receptor agonists and other antiobesity medications following bariatric surgery
- PMID: 38934217
- PMCID: PMC12223922
- DOI: 10.1111/dom.15737
Adverse event comparison between glucagon-like peptide-1 receptor agonists and other antiobesity medications following bariatric surgery
Abstract
Aim: To compare the incidence of adverse events (AEs) related to antiobesity medications (AOMs; glucagon-like peptide-1 receptor agonists [GLP-1RAs] vs. non-GLP-1RAs) after bariatric surgery.
Methods: This single-centre retrospective cohort included patients (aged 16-65 years) who had undergone laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy (cohort entry date) and initiated AOMs. Participants were categorized as users of US Food and Drug Administration (FDA)-approved, off-label, or GLP-1RA AOMs if documented as receiving the medication on or after cohort entry date. Non-GLP-1RA AOMs were phentermine, orlistat, topiramate, canagliflozin, dapagliflozin, empagliflozin, naltrexone, bupropion/naltrexone and phentermine/topiramate. GLP-1RA AOMs included: semaglutide, dulaglutide, exenatide and liraglutide. The primary outcome was AE incidence. Logistic regression was used to determine the association of AOM exposure with AEs.
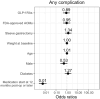
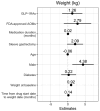
Results: We identified 599 patients meeting our inclusion criteria, 83% of whom were female. Their median (interquartile range [IQR]) age was 47.8 (40.9-55.4) years. The median duration of surgery to AOM exposure was 30 months. GLP-1RAs use was not associated with higher odds of AEs: adjusted odds ratio (aOR) 1.1 (95% confidence interval [CI] 0.5-2.6) and aOR 1.1 (95% CI 0.6-2.3) for GLP-1RA versus FDA-approved and off-label AOM use, respectively. AOM initiation ≥12 months after surgery was associated with lower risk of AEs compared to <12 months (aOR 0.01 [95% CI 0.0-0.01]; p < 0.001).
Conclusion: Our results showed that GLP-1RA AOMs were not associated with an increased risk of AEs compared to non-GLP-1RA AOMs in patients who had previously undergone bariatric surgery. Prospective studies are needed to identify the optimal timeframe for GLP-1RA initiation.
Keywords: adjuvant weight loss therapy; bariatric surgery; glucagon‐like peptide‐1 receptor agonists; inadequate weight loss.
© 2024 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflicts of interests.
Figures
References
-
- O'Brien PE, Hindle A, Brennan L, et al. Long‐term outcomes after bariatric surgery: a systematic review and meta‐analysis of weight loss at 10 or more years for all bariatric procedures and a single‐Centre review of 20‐year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3‐14. doi: 10.1007/s11695-018-3525-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

