Successful treatment with guanfacine in a long-COVID case manifesting marked cognitive impairment
- PMID: 38934345
- PMCID: PMC11544435
- DOI: 10.1002/npr2.12466
Successful treatment with guanfacine in a long-COVID case manifesting marked cognitive impairment
Abstract
Background: Persistent cognitive impairment is a serious consequence of the post-COVID condition. However, there have been no established effective treatments for this pathophysiology supported by sufficient evidence.
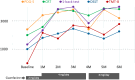
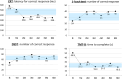
Case presentation: A 32-year-old woman became aware of difficulty in word recalling, reading, and writing as well as difficulty in completing various household multitasks 3 weeks after the COVID-19 infection. Although blood tests, magnetic resonance imaging, electroencephalography, and Kohs block design test were all within normal limits, completion time by trail making test (TMT) A or B was markedly delayed. Finally, she was referred to our hospital 3 months after the infection. At baseline, the THINC integrated tool (THINC-it), a digital battery consisting of the five-item version of the perceived deficit questionnaire (PDQ-5), choice reaction time (CRT), 1-back test, digit symbol substitution test (DSST), and TMT-B, revealed poor capability in attention, working memory, and executive function. Also, near-infrared spectroscopy (NIRS) demonstrated no activation in frontal or temporal regions during verbal fluency task. Extended-release guanfacine (GXR) 2 mg/day was initiated and a month later was elevated up to 4 mg/day as a maintenance dose. The PDQ-5, CRT, 1-back test, DSST, and TMT-B were dramatically improved 1 month after GXR treatment. NIRS finding was also normalized after 2 months of treatment. These effects were successfully maintained throughout the 6-month follow-up period.
Conclusion: GXR may be helpful in improving subjective/objective cognitive functioning and frontotemporal brain activity in long-COVID patients manifesting apparent cognitive impairment.
Keywords: THINC integrated tool; cognitive impairment; guanfacine; long COVID; near‐infrared spectroscopy.
© 2024 The Author(s). Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Society of Neuropsychopharmacology.
Conflict of interest statement
The authors declare they have no known competing financial interests or personal relationships that influenced the work reported in this study.
Figures

Similar articles
-
Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder.J Child Adolesc Psychopharmacol. 2011 Apr;21(2):111-20. doi: 10.1089/cap.2010.0064. Epub 2011 Apr 10. J Child Adolesc Psychopharmacol. 2011. PMID: 21476931 Clinical Trial.
-
Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial.Eur Neuropsychopharmacol. 2014 Dec;24(12):1861-72. doi: 10.1016/j.euroneuro.2014.09.014. Epub 2014 Oct 23. Eur Neuropsychopharmacol. 2014. PMID: 25453486 Clinical Trial.
-
Efficacy and Safety of Guanfacine Extended-Release in the Treatment of Attention-Deficit/Hyperactivity Disorder in Adults: Results of a Randomized, Double-Blind, Placebo-Controlled Study.J Clin Psychiatry. 2020 Apr 14;81(3):19m12979. doi: 10.4088/JCP.19m12979. J Clin Psychiatry. 2020. PMID: 32297719 Clinical Trial.
-
Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder.Adv Ther. 2012 May;29(5):385-400. doi: 10.1007/s12325-012-0020-1. Epub 2012 May 18. Adv Ther. 2012. PMID: 22610723 Review.
-
Evaluation of the current data on guanfacine extended release for the treatment of ADHD in children and adolescents.Expert Opin Pharmacother. 2020 Mar;21(4):417-426. doi: 10.1080/14656566.2019.1706480. Epub 2020 Jan 23. Expert Opin Pharmacother. 2020. PMID: 31971448 Review.
Cited by
-
Cognitive Sequelae of COVID-19: Mechanistic Insights and Therapeutic Approaches.CNS Neurosci Ther. 2025 Mar;31(3):e70348. doi: 10.1111/cns.70348. CNS Neurosci Ther. 2025. PMID: 40152069 Free PMC article. Review.
-
Case series: Potential use of guanfacine for safer management of behavioral disturbances in patients with dementia.PCN Rep. 2025 May 19;4(2):e70122. doi: 10.1002/pcn5.70122. eCollection 2025 Jun. PCN Rep. 2025. PMID: 40395628 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

