Functionality integration in stereolithography 3D printed microfluidics using a "print-pause-print" strategy
- PMID: 38934387
- PMCID: PMC11235415
- DOI: 10.1039/d4lc00147h
Functionality integration in stereolithography 3D printed microfluidics using a "print-pause-print" strategy
Erratum in
-
Correction: Functionality integration in stereolithography 3D printed microfluidics using a "print-pause-print" strategy.Lab Chip. 2025 Apr 29;25(9):2304. doi: 10.1039/d5lc90036k. Lab Chip. 2025. PMID: 40181757 Free PMC article.
Abstract
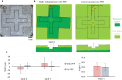
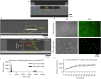
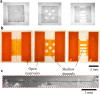
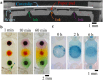
Stereolithography 3D printing, although an increasingly used fabrication method for microfluidic chips, has the main disadvantage of producing monolithic chips in a single material. We propose to incorporate during printing various objects using a "print-pause-print" strategy. Here, we demonstrate that this novel approach can be used to incorporate glass slides, hydrosoluble films, paper pads, steel balls, elastic or nanoporous membranes and silicon-based microdevices, in order to add microfluidic functionalities as diverse as valves, fluidic diodes, shallow chambers, imaging windows for bacteria tracking, storage of reagents, blue energy harvesting or filters for cell capture and culture.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures



References
-
- Accardo A. Courson R. Riesco R. Raimbault V. Malaquin L. Addit. Manuf. 2018;22:440–446.
LinkOut - more resources
Full Text Sources

