Pure Apocrine Intraductal Carcinoma of Salivary Glands: Reassessment of Molecular Underpinnings and Behavior
- PMID: 38935197
- PMCID: PMC11211294
- DOI: 10.1007/s12105-024-01653-2
Pure Apocrine Intraductal Carcinoma of Salivary Glands: Reassessment of Molecular Underpinnings and Behavior
Abstract
Background: Intraductal carcinoma (IDC) of the salivary glands is a confounding entity, our understanding of which continues to evolve. At least four forms have been elucidated based on histomorphology, immunophenotype, and molecular profile: (1) intercalated duct-like, S100/SOX10+ with frequent NCOA4::RET fusions; (2) oncocytic, S100/SOX10+ with TRIM33::RET, NCOA4::RET, and BRAF V600E; (3) apocrine, AR+ with PI3 kinase pathway mutations; and (4) mixed/hybrid intercalated duct-like/apocrine, with S100/SOX10+ and AR+ areas and frequent TRIM27::RET. The revelation that myoepithelial cells harbor the same fusion as luminal cells suggested that fusion-positive cases are not in situ carcinomas as previously believed. To this point, purely apocrine IDC with entirely intraductal growth has not been found to harbor fusions, but very few cases have been tested.
Methods: IDCs with pure apocrine morphology, entirely intraductal growth, and no precursor lesion (pleomorphic adenoma or sclerosing polycystic adenoma) were retrieved from the authors' archives. Several immunostains (S100, SOX10, GCDFP-15, AR, p40/SMA) and targeted next generation sequencing (NGS) panel including 1425 cancer-related genes were performed.
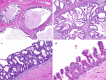
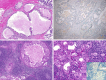
Results: Seven entirely IDC with pure apocrine type were collected. The cases arose in the parotid glands (mean, 1.9 cm) of 5 men and 2 women ranging from 51 to 84 years (mean, 69.7 years). Histologically, tumors consisted of rounded to angulated ductal cysts lined by epithelial cells with abundant finely granular eosinophilic cytoplasm and large nuclei with prominent nucleoli. Pleomorphism was mild to moderate, the mitotic rate was low, and necrosis was absent. Conventionally invasive foci or areas of intercalated duct-like morphology were not identified. In all cases, luminal cells were diffusely positive for AR and GCDFP-15 while negative for S100/SOX10, and the ducts were completely surrounded by myoepithelial cells highlighted by p40 and SMA. Molecular analysis was successful in 6 cases. Three harbored fusions: one with NCOA4::RET, another with STRN::ALK and one with both CDKN2A::CNTRL and TANC1::YY1AP1. The three fusion-negative cases all harbored HRAS mutations; additional mutations (PIK3CA, SPEN, ATM) were found in 2 of 3 cases. All patients were treated by surgery alone. Six of them are currently free of disease (follow up 12-190 months), but the case harboring NCOA4::RET developed lymph nodes metastasis in the form of a fusion-positive invasive salivary duct carcinoma.
Conclusions: Purely apocrine IDC is a heterogeneous disease. A subset seems to be genetically similar to salivary duct carcinoma and may indeed represent carcinoma in situ. The other group harbors fusions, similar to other forms of IDC. Moreover, the occurrence of lymph node metastasis discredits the idea that any fusion-positive IDC with a complete myoepithelial cell layer has no metastatic potential. With the wide use of RET-and ALK-based targeted therapies, our findings further underscore the importance of fusion analysis for IDC.
Keywords: NCOA4::RET; STRN::ALK; Intraductal carcinoma; Salivary duct carcinoma; Salivary gland neoplasms.
© 2024. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Brandwein-Gensler M, Gnepp DR (2005) Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) Pathology and genetics of head and neck tumours. World Health Organization classification of tumours, Lyon
-
- Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ et al (2004) Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol 28(8):1040–1044 - PubMed
-
- Chen KT (1983) Intraductal carcinoma of the minor salivary gland. J Laryngol Otol 97(2):189–191 - PubMed
-
- Delgado R, Klimstra D, Albores-Saavedra J (1996) Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer 78(5):958–967 - PubMed
-
- Loening T, Leivo I, Simpson RHW, Weinreb I (2017) Intraductal carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ (eds) WHO Classification of Head and Neck Tumours. International Agency for Research on Cancer, Lyon, pp 170–171
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

