Polyaromatic Calixarene Hosts: Calix[4]pyrenes
- PMID: 38935556
- PMCID: PMC11249777
- DOI: 10.1021/acs.orglett.4c01850
Polyaromatic Calixarene Hosts: Calix[4]pyrenes
Abstract
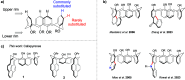
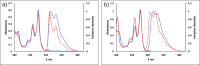
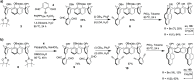
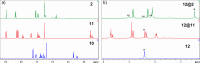
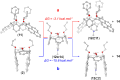
Calixpyrenes, calix[4]arenes incorporating one or two pyrene moieties as a part of their hydrophobic cavities, have been prepared and fully characterized. Distally di-O-propoxy diether of the calix dipyrene, which exists in the pinched cone conformation with nearly parallel pyrene moieties, demonstrates strongly enhanced binding of an organic cation (N-methylpyridinium) compared with the analogous diethers of the parent calix[4]arene.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
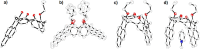
References
-
- Gutsche C. D.Calixarenes Revisited; The Royal Society of Chemistry: Cambridge, U.K., 1998.
- Dalla Cort A.; Mandolini L.. Calixarenes in Action; Mandolini L., Ungaro R., Eds.; Imperial College Press: London, U.K., 2000.
- Abraham W. Inclusion of Organic Cations by Calix[n]arenes. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 159–174. 10.1023/A:1021288303104. - DOI
-
- Gutsche C. D. Calixarenes. Acc. Chem. Res. 1983, 16, 161–170. 10.1021/ar00089a003. - DOI
- Vicens J.; Böhmer V.. Calixarenes: A Versatile Class of Macrocyclic Compounds; Springer: Dordrecht, The Netherlands, 1990.
-
- Ogoshi T.; Yamagishi T. Pillararenes: Versatile Synthetic Receptors for Supramolecular Chemistry. Eur. J. Org. Chem. 2013, 2013, 2961–2975. 10.1002/ejoc.201300079. - DOI
-
- Georghiou P. E.; Ashram M.; Li Z.; Chaulk S. G. Syntheses of Calix[4]naphthalenes Derived from 1-Naphthol. J. Org. Chem. 1995, 60, 7284–7289. 10.1021/jo00127a038. - DOI
LinkOut - more resources
Full Text Sources

