Golgi associated RAB2 interactor protein family contributes to murine male fertility to various extents by assuring correct morphogenesis of sperm heads
- PMID: 38935810
- PMCID: PMC11236154
- DOI: 10.1371/journal.pgen.1011337
Golgi associated RAB2 interactor protein family contributes to murine male fertility to various extents by assuring correct morphogenesis of sperm heads
Abstract
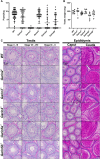
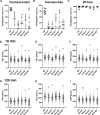
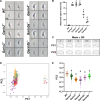
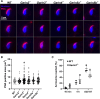
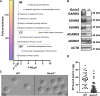
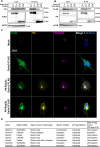
Sperm heads contain not only the nucleus but also the acrosome which is a distinctive cap-like structure located anterior to the nucleus and is derived from the Golgi apparatus. The Golgi Associated RAB2 Interactors (GARINs; also known as FAM71) protein family shows predominant expression in the testis and all possess a RAB2-binding domain which confers binding affinity to RAB2, a small GTPase that is responsible for membrane transport and vesicle trafficking. Our previous study showed that GARIN1A and GARIN1B are important for acrosome biogenesis and that GARIN1B is indispensable for male fertility in mice. Here, we generated KO mice of other Garins, namely Garin2, Garin3, Garin4, Garin5a, and Garin5b (Garin2-5b). Using computer-assisted morphological analysis, we found that the loss of each Garin2-5b resulted in aberrant sperm head morphogenesis. While the fertilities of Garin2-/- and Garin4-/- males are normal, Garin5a-/- and Garin5b-/- males are subfertile, and Garin3-/- males are infertile. Further analysis revealed that Garin3-/- males exhibited abnormal acrosomal morphology, but not as severely as Garin1b-/- males; instead, the amounts of membrane proteins, particularly ADAM family proteins, decreased in Garin3 KO spermatozoa. Moreover, only Garin4 KO mice exhibit vacuoles in the sperm head. These results indicate that GARINs assure correct head morphogenesis and some members of the GARIN family function distinctively in male fertility.
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

