Paediatric strategy forum for medicinal product development of PI3-K, mTOR, AKT and GSK3β inhibitors in children and adolescents with cancer
- PMID: 38936103
- PMCID: PMC12393138
- DOI: 10.1016/j.ejca.2024.114145
Paediatric strategy forum for medicinal product development of PI3-K, mTOR, AKT and GSK3β inhibitors in children and adolescents with cancer
Abstract
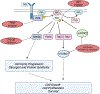
Phosphatidylinositol 3-kinase (PI3-K) signalling pathway is a crucial path in cancer for cell survival and thus represents an intriguing target for new paediatric anti-cancer drugs. However, the unique clinical toxicities of targeting this pathway (resulting in hyperglycaemia) difficulties combining with chemotherapy, rarity of mutations in childhood tumours and concomitant mutations have resulted in major barriers to clinical translation of these inhibitors in treating both adults and children. Mutations in PIK3CA predict response to PI3-K inhibitors in adult cancers. The same mutations occur in children as in adults, but they are significantly less frequent in paediatrics. In children, high-grade gliomas, especially diffuse midline gliomas (DMG), have the highest incidence of PIK3CA mutations. New mutation-specific PI3-K inhibitors reduce toxicity from on-target PI3-Kα wild-type activity. The mTOR inhibitor everolimus is approved for subependymal giant cell astrocytomas. In paediatric cancers, mTOR inhibitors have been predominantly evaluated by academia, without an overall strategy, in empiric, mutation-agnostic clinical trials with very low response rates to monotherapy. Therefore, future trials of single agent or combination strategies of mTOR inhibitors in childhood cancer should be supported by very strong biological rationale and preclinical data. Further preclinical evaluation of glycogen synthase kinase-3 beta inhibitors is required. Similarly, even where there is an AKT mutation (∼0.1 %), the role of AKT inhibitors in paediatric cancers remains unclear. Patient advocates strongly urged analysing and conserving data from every child participating in a clinical trial. A priority is to evaluate mutation-specific, central nervous system-penetrant PI3-K inhibitors in children with DMG in a rational biological combination. The choice of combination, should be based on the genomic landscape e.g. PTEN loss and resistance mechanisms supported by preclinical data. However, in view of the very rare populations involved, innovative regulatory approaches are needed to generate data for an indication.
Keywords: AKT and GSK3β Inhibitors; Cancer therapeutics; Combinations; Diffuse midline glioma; Drug development; MTOR; PI3-K; Paediatric oncology; Paediatric strategy forum.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest RB is an employee of Genentech, A Member of the Roche Group, South San Francisco, CA USA. JC is an employee of Bayer Healthcare Pharmaceuticals, Whippany, NJ, USA. SGD has received consulting fees for advisory board participation from Amgen, Bayer, InhibRx, and Jazz and travel funding from Loxo, Roche, and Salarius. JF is an employee of Kazia Therapeutics. IG an employee of Celcuity. HK is an employee of Loxo Oncology at Lilly. DL is an employee of Merck Sharp & Dohme. AM is an employee of Actuate. MEM has receiving consulting fees for advisory board participation from Ymabs Therapeutics, Recordati and travel funding from Bayer. KN has received consulting fees for advisory board participation from Y-mAbs, EUSA Pharma and Bayer, fees for teaching from Bayer and serving on a data monitoring committee from Lilly. ADJP has consulted for Lilly, Norgine and Developmental Therapeutics Consortium Limited and been an advisor for Amgen. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol.2018; 15:273–291. - PubMed
-
- Combination Chemotherapy With or Without Temsirolimus in Treating Patients with Intermediate Risk Rhabdomyosarcoma. https://clinicaltrials.gov/study/NCT02567435. (Accessed 19 November 2023)
-
- Efficacy and Safety of Everolimus (RAD001) in Patients of All Ages With Subependymal Giant Cell Astrocytoma Associated With Tuberous Sclerosis Complex (TSC) (EXIST-1). https://clinicaltrials.gov/study/NCT00789828. (Accessed 19 November 2023)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

