NADPH Alters DUOX1 Calcium Responsiveness
- PMID: 38936256
- PMCID: PMC11259916
- DOI: 10.1016/j.redox.2024.103251
NADPH Alters DUOX1 Calcium Responsiveness
Abstract
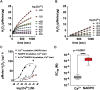
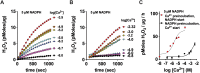
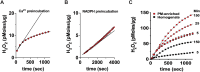
Hydrogen peroxide is a key element in redox signaling and in setting cellular redox tone. DUOX1 and DUOX2, that directly synthesize hydrogen peroxide, are the most abundant NADPH oxidase transcripts in most epithelia. DUOX1 and DUOX2 hydrogen peroxide synthesis is regulated by intracellular calcium transients and thus cells can respond to signals and initiate responses by increasing cellular hydrogen peroxide synthesis. Nevertheless, many details of their enzymatic regulation are still unexplored. DUOX1 and DUOXA1 were expressed in HEK293T cells and activity was studied in homogenates and membrane fractions. When DUOX1 homogenates or membranes were pre-incubated in NADPH and started with addition of Ca2+, to mimic intracellular activation, progress curves were distinctly different from those pre-incubated in Ca2+ and started with NADPH. The Ca2+ EC50 for DUOX1's initial rate when pre-incubated in Ca2+, was three orders of magnitude lower (EC50 ∼ 10-6 M) than with preincubation in NADPH (EC50 ∼ 10-3 M). In addition, activity was several fold lower with Ca2+ start. Identical results were obtained using homogenates and membrane fractions. The data suggested that DUOX1 Ca2+ binding in expected physiological signaling conditions only slowly leads to maximal hydrogen peroxide synthesis and that full hydrogen peroxide synthesis activity in vivo only can occur when encountering extremely high concentration Ca2+ signals. Thus, a complex interplay of intracellular NADPH and Ca2+ concentrations regulate DUOX1 over a wide extent and may limit DUOX1 activity to a restricted range and spatial distribution.
Keywords: DUOX; Hydrogen peroxide; NADPH oxidase.
Copyright © 2024 The Author. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Rigutto S., Hoste C., Grasberger H., Milenkovic M., Communi D., Dumont J.E., Corvilain B., Miot F., De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009;284(11):6725–6734. PubMed PMID: 19144650. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

