Exploring xylose metabolism in non-conventional yeasts: kinetic characterization and product accumulation under different aeration conditions
- PMID: 38936832
- PMCID: PMC11247345
- DOI: 10.1093/jimb/kuae023
Exploring xylose metabolism in non-conventional yeasts: kinetic characterization and product accumulation under different aeration conditions
Abstract
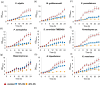
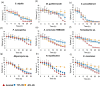
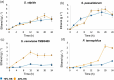
d-Xylose is a metabolizable carbon source for several non-Saccharomyces species, but not for native strains of S. cerevisiae. For the potential application of xylose-assimilating yeasts in biotechnological processes, a deeper understanding of pentose catabolism is needed. This work aimed to investigate the traits behind xylose utilization in diverse yeast species. The performance of 9 selected xylose-metabolizing yeast strains was evaluated and compared across 3 oxygenation conditions. Oxygenation diversely impacted growth, xylose consumption, and product accumulation. Xylose utilization by ethanol-producing species such as Spathaspora passalidarum and Scheffersomyces stipitis was less affected by oxygen restriction compared with other xylitol-accumulating species such as Meyerozyma guilliermondii, Naganishia liquefaciens, and Yamadazyma sp., for which increased aeration stimulated xylose assimilation considerably. Spathaspora passalidarum exhibited superior conversion of xylose to ethanol and showed the fastest growth and xylose consumption in all 3 conditions. By performing assays under identical conditions for all selected yeasts, we minimize bias in comparisons, providing valuable insight into xylose metabolism and facilitating the development of robust bioprocesses.
One-sentence summary: This work aims to expand the knowledge of xylose utilization in different yeast species, with a focus on how oxygenation impacts xylose assimilation.
Keywords: Bioethanol; Kinetic characterization; Xylitol; Xylose utilization, Non-conventional yeasts.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Conflict of interest statement
The authors declare no conflicts of interest and no competing financial interests.
Figures


Similar articles
-
Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum NRRL Y-27907 and Scheffersomyces stipitis NRRL Y-7124.Biotechnol Bioeng. 2015 Mar;112(3):457-69. doi: 10.1002/bit.25445. Biotechnol Bioeng. 2015. PMID: 25164099
-
Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae.Biotechnol Biofuels. 2016 Aug 5;9:167. doi: 10.1186/s13068-016-0570-6. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27499810 Free PMC article.
-
Diversity and physiological characterization of D-xylose-fermenting yeasts isolated from the Brazilian Amazonian Forest.PLoS One. 2012;7(8):e43135. doi: 10.1371/journal.pone.0043135. Epub 2012 Aug 13. PLoS One. 2012. PMID: 22912807 Free PMC article.
-
Genetic improvement of native xylose-fermenting yeasts for ethanol production.J Ind Microbiol Biotechnol. 2015 Jan;42(1):1-20. doi: 10.1007/s10295-014-1535-z. Epub 2014 Nov 18. J Ind Microbiol Biotechnol. 2015. PMID: 25404205 Review.
-
Metabolic engineering for improved fermentation of pentoses by yeasts.Appl Microbiol Biotechnol. 2004 Feb;63(5):495-509. doi: 10.1007/s00253-003-1450-0. Epub 2003 Nov 1. Appl Microbiol Biotechnol. 2004. PMID: 14595523 Review.
Cited by
-
Assessing Process Conditions on Xylose Fermentation in Spathaspora passalidarum: Effects of pH, Substrate-to-Inoculum Ratio, Temperature, and Initial Ethanol Concentration.Curr Microbiol. 2024 Nov 7;81(12):448. doi: 10.1007/s00284-024-03976-3. Curr Microbiol. 2024. PMID: 39508833
References
-
- Baptista S. L., Romaní A., Domingues L. (2021). Biotechnological advancements, innovations and challenges for sustainable xylitol production by yeast. In Encyclopedia of Mycology (pp. 420–427). Elsevier. 10.1016/b978-0-12-809633-8.21580-x - DOI
-
- Barros K. O., Mader M., Krause D. J., Pangilinan J., Andreopoulos B., Lipzen A., Mondo S. J., Grigoriev I. V., Rosa C. A., Sato T. K., Hittinger C. T. (2024). Oxygenation influences xylose fermentation and gene expression in the yeast genera Spathaspora and Scheffersomyces. Biotechnology for Biofuels and Bioproducts, 17(1), 20. 10.1186/s13068-024-02467-8 - DOI - PMC - PubMed
-
- Basso T. P., Procópio D. P., Petrin T. H. C., Giacon T. G., Jin Y. S., Basso T. O., Basso L. C. (2023). Engineering xylose fermentation in an industrial yeast: Continuous cultivation as a tool for selecting improved strains. Letters in Applied Microbiology, 76(7). 10.1093/lambio/ovad077 - DOI - PubMed
-
- Bonan C. I. D. G., Tramontina R., dos Santos M. W., Biazi L. E., Soares L. B., Pereira I. O., Hoffmam Z. B., Coutouné N., Squina F. M., Robl D., Ienczak J. L. (2022). Biorefinery platform for Spathaspora passalidarum NRRL Y-27907 in the production of ethanol, xylitol, and single cell protein from sugarcane bagasse. BioEnergy Research, 15(2), 1169–1181. 10.1007/s12155-021-10255-7 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

