Long-term impact of ivacaftor on mortality rate and health outcomes in people with cystic fibrosis
- PMID: 38937105
- PMCID: PMC11503052
- DOI: 10.1136/thorax-2023-220558
Long-term impact of ivacaftor on mortality rate and health outcomes in people with cystic fibrosis
Abstract
Background: Ivacaftor (IVA) has been shown to improve lung function and other clinical outcomes in people with cystic fibrosis (CF). A decade of real-world IVA availability has enabled the examination of long-term outcomes with this treatment. This retrospective, longitudinal cohort study investigated the impact of IVA on mortality rate and health outcomes among people with CF in the US.
Methods: Data from the US CF Foundation Patient Registry from January 2010 to December 2019 were analysed. The IVA-treated cohort included people with a CF transmembrane conductance regulator (CFTR) gating mutation (excluding R117H); age-matched comparator cohort included people with a F508del and a minimal function CFTR mutation who had no prior CFTR modulator treatment. Baseline characteristics were balanced between cohorts using standardised mortality ratio weighting generated from propensity scores. Outcomes of interest were overall survival, lung transplant, percent predicted forced expiratory volume in 1 s (ppFEV1), body mass index (BMI), pulmonary exacerbations (PEx), outpatient visits and hospitalisations.
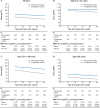
Findings: Over a maximum follow-up of 7.9 years, the IVA-treated cohort (N=736) had lower rates of mortality (hazard ratio [HR] (95% CI): 0.22 (0.09 to 0.45)), lung transplant (HR: 0.11 (95% CI 0.02 to 0.28)), PEx (rate ratio: 0.49 (95% CI 0.42 to 0.55)) and all-cause hospitalisations (rate ratio: 0.50 (95% CI 0.43 to 0.56)) as well as better lung function (mean difference in ppFEV1: 8.46 (95% CI 7.34 to 9.75)) and higher BMI/BMI z-scores (mean difference 1.20 (95% CI 0.92 to 1.71) kg/m2 and 0.27 (95% CI 0.25 to 0.40), respectively) than the comparator cohort (N=733).
Interpretation: Our analysis suggests that IVA provides sustained clinical benefits in people with CF over a follow-up period of approximately 8 years. These findings reinforce the existing real-world evidence that IVA can slow disease progression and decrease the healthcare burden of CF over the long term.
Keywords: Cystic Fibrosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors received non-financial support (assistance with manuscript preparation) from ArticulateScience, which was funded by Vertex Pharmaceuticals. CAM reports funding from Vertex Pharmaceuticals. MD, CN and YMG are employees of Analysis Group, which received research funding/consultancy fees from Vertex Pharmaceuticals. TT, LJM, JH and JLR are current or former employees of Vertex Pharmaceuticals and may own stock or stock options in that company. MAB has served as a scientific advisory committee member for Amgen, Astellas/Seagen, Atara Biotherapeutics, Brigham and Women’s Hospital, Kite, Gilead, Intercept, NIDDK, and Vertex Pharmaceuticals and has consulting fees/equity with Target RWE and equity with Accompany Health.
Figures



Similar articles
-
Impact of age at ivacaftor initiation on pulmonary outcomes among people with cystic fibrosis.Thorax. 2024 Sep 18;79(10):915-924. doi: 10.1136/thorax-2023-220559. Thorax. 2024. PMID: 38719441 Free PMC article.
-
LONGITUDE: An observational study of the long-term effectiveness of elexacaftor/tezacaftor/ivacaftor in people aged ≥12 years with cystic fibrosis using data from the United Kingdom Cystic Fibrosis Registry - 2-year analysis.J Cyst Fibros. 2025 Jul;24(4):716-723. doi: 10.1016/j.jcf.2025.04.012. Epub 2025 May 15. J Cyst Fibros. 2025. PMID: 40379539
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2015 Mar 26;(3):CD009841. doi: 10.1002/14651858.CD009841.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 07;1:CD009841. doi: 10.1002/14651858.CD009841.pub3. PMID: 25811419 Updated.
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. doi: 10.1002/14651858.CD010966.pub4. Cochrane Database Syst Rev. 2023. PMID: 37983082 Free PMC article.
-
Impact of Cystic Fibrosis Transmembrane Conductance Regulator Modulators on Maternal Outcomes During and After Pregnancy.Chest. 2025 Feb;167(2):348-361. doi: 10.1016/j.chest.2024.09.019. Epub 2024 Sep 27. Chest. 2025. PMID: 39343292
Cited by
-
Evolving nutrition therapy in cystic fibrosis: Adapting to the CFTR modulator era.Nutr Clin Pract. 2025 Aug;40(4):816-828. doi: 10.1002/ncp.11332. Epub 2025 Jun 18. Nutr Clin Pract. 2025. PMID: 40533897 Free PMC article. Review.
-
A Retrospective, Longitudinal Registry Study on the Long-Term Durability of Ivacaftor Treatment in People with Cystic Fibrosis.Pulm Ther. 2024 Dec;10(4):483-494. doi: 10.1007/s41030-024-00269-9. Epub 2024 Sep 12. Pulm Ther. 2024. PMID: 39266929 Free PMC article.
-
Impact of age at ivacaftor initiation on pulmonary outcomes among people with cystic fibrosis.Thorax. 2024 Sep 18;79(10):915-924. doi: 10.1136/thorax-2023-220559. Thorax. 2024. PMID: 38719441 Free PMC article.
-
Impact of CFTR Modulators on Longitudinal Cystic Fibrosis Survival and Mortality: Review and Secondary Analysis.Pulm Ther. 2025 Sep;11(3):365-386. doi: 10.1007/s41030-025-00303-4. Epub 2025 Jul 11. Pulm Ther. 2025. PMID: 40646419 Free PMC article. Review.
References
-
- Patient Registry 2021 Annual Data Report . Cystic Fibrosis Foundation. Bethesda, MD: 2022.
-
- European Cystic Fibrosis Society Patient Registry 2020 Annual Data Report. 2022. [6-Dec-2022]. https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports Available. Accessed.
-
- Cystic Fibrosis Canada The Canadian Cystic Fibrosis Registry 2021 Annual Data Report. [15-Nov-2023]. https://www.cysticfibrosis.ca/registry/2021AnnualDataReport.pdf Available. Accessed.
-
- Cystic Fibrosis Australia Australian Cystic Fibrosis Data Registry Annual Report. 2021. [15-Nov-2023]. https://cysticfibrosis.org.au/wp-content/uploads/2023/05/2021-ACFDR-Annu... Available. Accessed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
