Single-cell transcriptomic profiling reveals decreased ER protein Reticulon3 drives the progression of renal fibrosis
- PMID: 38937317
- PMCID: PMC11211315
- DOI: 10.1186/s43556-024-00187-x
Single-cell transcriptomic profiling reveals decreased ER protein Reticulon3 drives the progression of renal fibrosis
Abstract
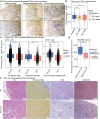
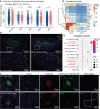
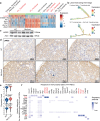
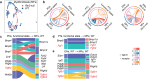
Chronic kidney disease (CKD) poses a significant global health dilemma, emerging from complex causes. Although our prior research has indicated that a deficiency in Reticulon-3 (RTN3) accelerates renal disease progression, a thorough examination of RTN3 on kidney function and pathology remains underexplored. To address this critical need, we generated Rtn3-null mice to study the consequences of RTN3 protein deficiency on CKD. Single-cell transcriptomic analyses were performed on 47,885 cells from the renal cortex of both healthy and Rtn3-null mice, enabling us to compare spatial architectures and expression profiles across 14 distinct cell types. Our analysis revealed that RTN3 deficiency leads to significant alterations in the spatial organization and gene expression profiles of renal cells, reflecting CKD pathology. Specifically, RTN3 deficiency was associated with Lars2 overexpression, which in turn caused mitochondrial dysfunction and increased reactive oxygen species levels. This shift induced a transition in renal epithelial cells from a functional state to a fibrogenic state, thus promoting renal fibrosis. Additionally, RTN3 deficiency was found to drive the endothelial-to-mesenchymal transition process and disrupt cell-cell communication, further exacerbating renal fibrosis. Immunohistochemistry and Western-Blot techniques were used to validate these observations, reinforcing the critical role of RTN3 in CKD pathogenesis. The deficiency of RTN3 protein in CKD leads to profound changes in cellular architecture and molecular profiles. Our work seeks to elevate the understanding of RTN3's role in CKD's narrative and position it as a promising therapeutic contender.
Keywords: Cell-cell communication; Chronic kidney disease (CKD); Endothelial-to-mesenchymal transition (EndoMT); Reactive oxygen species (ROS); Reticulon-3; Single-cell transcriptomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 82170598, 82270750, 82270730, 82070738, 81970403, 82300787 and 82000427/National Natural Science Foundation of China
- 2022sk2034/Key Research and Development Program of Hunan Province
- 2022JJ30058, 2021JJ40992 and 2023JJ20078/Natural Science Foundation of Hunan Province
- 2020TQ0363 and 2020M682598/China Postdoctoral Science Foundation
- 2021Q11/Youth Science Foundation of Xiangya Hospital
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
