Facile fabrication of recyclable robust noncovalent porous crystals from low-symmetry helicene derivative
- PMID: 38937477
- PMCID: PMC11211482
- DOI: 10.1038/s41467-024-49865-y
Facile fabrication of recyclable robust noncovalent porous crystals from low-symmetry helicene derivative
Abstract
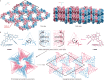
Porous frameworks constructed via noncovalent interactions show wide potential in molecular separation and gas adsorption. However, it remains a major challenge to prepare these materials from low-symmetry molecular building blocks. Herein, we report a facile strategy to fabricate noncovalent porous crystals through modular self-assembly of a low-symmetry helicene racemate. The P and M enantiomers in the racemate first stack into right- and left-handed triangular prisms, respectively, and subsequently the two types of prisms alternatively stack together into a hexagonal network with one-dimensional channels with a diameter of 14.5 Å. Remarkably, the framework reveals high stability upon heating to 275 °C, majorly due to the abundant π-interactions between the complementarily engaged helicene building blocks. Such porous framework can be readily prepared by fast rotary evaporation, and is easy to recycle and repeatedly reform. The refined porous structure and enriched π-conjugation also favor the selective adsorption of a series of small molecules.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

