Human mitochondrial carriers of the SLC25 family function as monomers exchanging substrates with a ping-pong kinetic mechanism
- PMID: 38937634
- PMCID: PMC11329753
- DOI: 10.1038/s44318-024-00150-0
Human mitochondrial carriers of the SLC25 family function as monomers exchanging substrates with a ping-pong kinetic mechanism
Abstract
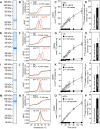
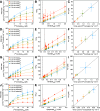
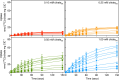
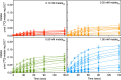
Members of the SLC25 mitochondrial carrier family link cytosolic and mitochondrial metabolism and support cellular maintenance and growth by transporting compounds across the mitochondrial inner membrane. Their monomeric or dimeric state and kinetic mechanism have been a matter of long-standing debate. It is believed by some that they exist as homodimers and transport substrates with a sequential kinetic mechanism, forming a ternary complex where both exchanged substrates are bound simultaneously. Some studies, in contrast, have provided evidence indicating that the mitochondrial ADP/ATP carrier (SLC25A4) functions as a monomer, has a single substrate binding site, and operates with a ping-pong kinetic mechanism, whereby ADP is imported before ATP is exported. Here we reanalyze the oligomeric state and kinetic properties of the human mitochondrial citrate carrier (SLC25A1), dicarboxylate carrier (SLC25A10), oxoglutarate carrier (SLC25A11), and aspartate/glutamate carrier (SLC25A13), all previously reported to be dimers with a sequential kinetic mechanism. We demonstrate that they are monomers, except for dimeric SLC25A13, and operate with a ping-pong kinetic mechanism in which the substrate import and export steps occur consecutively. These observations are consistent with a common transport mechanism, based on a functional monomer, in which a single central substrate-binding site is alternately accessible.
Keywords: Bioenergetics; Kinetic Analysis; Mitochondria; SLC25 Mitochondrial Carrier Family; Transport.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Alexander CG, Wanner R, Johnson CM, Breitsprecher D, Winter G, Duhr S, Baaske P, Ferguson N (2014) Novel microscale approaches for easy, rapid determination of protein stability in academic and commercial settings. Biochim Biophys Acta 1844:2241–2250 10.1016/j.bbapap.2014.09.016 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

