The development and implementation of a proficiency testing program for SARS-CoV-2 using dried tube specimens in resource-limited countries
- PMID: 38937708
- PMCID: PMC11209982
- DOI: 10.1186/s12879-024-09555-y
The development and implementation of a proficiency testing program for SARS-CoV-2 using dried tube specimens in resource-limited countries
Abstract
Introduction: When COVID-19 hit the world in 2019, an enhanced focus on diagnostic testing for SARS-CoV-2 was essential for a successful pandemic response. Testing laboratories stretched their capabilities for the new coronavirus by adopting different test methods. The necessity of having external quality assurance (EQA) mechanisms was even more critical due to this rapid expansion. However, there was a lack of experience in providing the necessary SARS-CoV-2 EQA materials, especially in locations with constrained resources.
Objective: We aimed to create a PT (Proficiency testing) programme based on the Dried Tube Specimens (DTS) method that would be a practical option for molecular based SARS-CoV-2 EQA in Low- and Middle-Income Countries.
Methods: Based on previous ISO/IEC 17043:2010 accreditation experiences and with assistance from the US Centers for Disease Control and Prevention, The Supranational Reference Laboratory of Uganda (adapted the DTS sample preparation method and completed a pilot EQA program between 2020 and 2021. Stability and panel validation testing was conducted on the designed materials before shipping to pilot participants in six African countries. Participants received a panel containing five SARS-CoV-2 DTS samples, transported at ambient conditions. Results submitted by participants were compared to validation results. Participants were graded as satisfactory (≥ 80%) or unsatisfactory (< 80%) and performance reports disseminated.
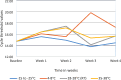
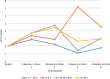
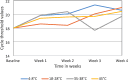
Results: Our SARS-CoV-2 stability experiments showed that SARS-CoV-2 RNA was stable (-15 to -25 °C, 4 to 8 °C, (18 to 28 °C) room temperature and 35 to 38 °C) as well as DTS panels (4 to 8 °C, 18 to 28 °C, 35 to 38 °C and 45 °C) for a period of 4 weeks. The SARS-CoV-2 DTS panels were successfully piloted in 35 test sites from Zambia, Malawi, Mozambique, Nigeria, and Seychelles. The pilot results of the participants showed good accuracy, with an average of 86% (30/35) concordance with the original SARS CoV-2 expectations.
Conclusion: The SARS-CoV-2 DTS PT panel is reliable, stable at ambient temperature, simple to prepare and requires minimal resources.
Keywords: Dried tube specimens; External quality assessment; Proficiency testing; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- World Health Organization (WHO). Coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_1 (Accessed Jan 17, 2022).
-
- World Health Organization (WHO). WHO COVID-19: case definitions: updated in public health surveillance for COVID-19, World Health Organization, 2020. [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/337834/WHO-2019-nCoV-Su....
-
- World Health Organization (WHO). Diagnostic testing for SARS-CoV-2: interim guidance, 11 September 2020. https://apps.who.int/iris/handle/10665/334254 (accessed Jan. 17, 2022).
-
- World Health Organization (WHO). Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities. https://www.who.int/publications/i/item/WHO-2019-nCoV-lab-testing-2021.1... (accessed Jan. 17, 2022).
-
- World Health Organization (WHO). Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. https://www.who.int/publications/i/item/10665-331501 (accessed Jan. 19, 2022).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

