Conformational rigidity of cytochrome c'-α from a thermophile is associated with slow NO binding
- PMID: 38937973
- PMCID: PMC11365222
- DOI: 10.1016/j.bpj.2024.06.026
Conformational rigidity of cytochrome c'-α from a thermophile is associated with slow NO binding
Abstract
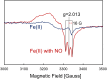
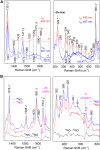
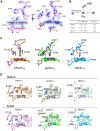
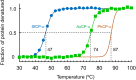
Cytochromes c'-α are nitric oxide (NO)-binding heme proteins derived from bacteria that can thrive in a wide range of temperature environments. Studies of mesophilic Alcaligenes xylosoxidans cytochrome c'-α (AxCP-α) have revealed an unusual NO-binding mechanism involving both heme faces, in which NO first binds to form a distal hexa-coordinate Fe(II)-NO (6cNO) intermediate and then displaces the proximal His to form a proximal penta-coordinate Fe(II)-NO (5cNO) final product. Here, we characterize a thermally stable cytochrome c'-α from thermophilic Hydrogenophilus thermoluteolus (PhCP-α) to understand how protein thermal stability affects NO binding. Electron paramagnetic and resonance Raman spectroscopies reveal the formation of a PhCP-α 5cNO product, with time-resolved (stopped-flow) UV-vis absorbance indicating the involvement of a 6cNO intermediate. Relative to AxCP-α, the rates of 6cNO and 5cNO formation in PhCP-α are ∼11- and ∼13-fold lower, respectively. Notably, x-ray crystal structures of PhCP-α in the presence and absence of NO suggest that the sluggish formation of the proximal 5cNO product results from conformational rigidity: the Arg-132 residue (adjacent to the proximal His ligand) is held in place by a salt bridge between Arg-75 and Glu-135 (an interaction not present in AxCP-α or a psychrophilic counterpart). Overall, our data provide fresh insights into structural factors controlling NO binding in heme proteins, including 5cNO complexes relevant to eukaryotic NO sensors.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Ambler R.P. Sequence variability in bacterial cytochromes c. Biochim. Biophys. Acta. 1991;1058:42–47. - PubMed
-
- Inoue H., Wakai S., et al. Sambongi Y. Heterologous synthesis of cytochrome c′ by Escherichia coli is not dependent on the System I cytochrome c biogenesis machinery. FEBS J. 2011;278:2341–2348. - PubMed
-
- Suka A., Oki H., et al. Sambongi Y. Stability of cytochromes c′ from psychrophilic and piezophilic Shewanella species: implications for complex multiple adaptation to low temperature and high hydrostatic pressure. Extremophiles. 2019;23:239–248. - PubMed
-
- Shibata N., Iba S., et al. Yasuoka N. Basis for monomer stabilization in Rhodopseudomonas palustris cytochrome c′ derived from the crystal structure. J. Mol. Biol. 1998;284:751–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

