Antidiabetic, antidyslipidemia, and renoprotector potency of butterfly pea flower extract (Clitorea ternatea L.) in diabetes mellitus and dyslipidemia rats model
- PMID: 38938424
- PMCID: PMC11199744
- DOI: 10.5455/OVJ.2024.v14.i5.7
Antidiabetic, antidyslipidemia, and renoprotector potency of butterfly pea flower extract (Clitorea ternatea L.) in diabetes mellitus and dyslipidemia rats model
Abstract
Background: Diabetes mellitus (DM) is a long-term condition marked by high blood glucose levels caused by insulin resistance which will lead to complications of other diseases such as dyslipidemia, which also affects the health of the liver and kidneys. Butterfly pea flower (Clitorea ternatea L.) has phenolic and flavonoid compounds which have the potential as herbal medicines for antidiabetics.
Aim: The purpose of this study is to examine the potential of butterfly pea flower extract (BPE) as an antidiabetic, anti-dyslipidemia, and renoprotection.
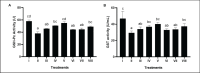
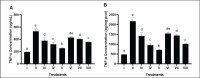
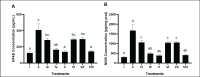
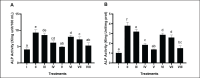
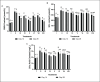
Methods: In vivo test was performed on Sprague Dawley rats (Rattus norvegicus L.) induced by Streptozotocin-Nicotinamide and High Fat Diet-Propylthiouracil as models of DM and dyslipidemia, and BPE was administered orally (200, 400, and 800 mg/kg BW) for 28 days. glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), tumor necrosis factor-α (TNF-α), nuclear factor-kappa beta (NF-kB), alkaline phosphatase (ALP), liver albumin levels, serum blood urea nitrogen (BUN), serum creatinine, and serum uric acid (UA), were measured by ELISA and colorimetry methods.
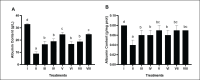
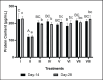
Results: Treatment of BPE 800 mg/kg BW increased levels of GSH-Px, GST, albumin, and serum protein. BPE decreased TNF-α, NF-kB, and ALP. BPE also decreased BUN, serum CR, and serum UA.
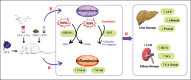
Conclusion: BPE has the potential to be used as a drug alternative for the treatment of DM and dyslipidemia as well as a hepatoprotective and renoprotective agent.
Keywords: Antidiabetic; Clitorea ternatea L.; Diabetes mellitus; Dyslipidemia; Renoprotective.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Antidiabetic and hepatoprotection effect of butterfly pea flower (Clitoria ternatea L.) through antioxidant, anti-inflammatory, lower LDH, ACP, AST, and ALT on diabetes mellitus and dyslipidemia rat.Heliyon. 2024 Apr 16;10(8):e29812. doi: 10.1016/j.heliyon.2024.e29812. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681657 Free PMC article.
-
Attenuation of hyperglycemia-associated dyslipidemic, oxidative, cognitive, and inflammatory crises via modulation of neuronal ChEs/NF-κB/COX-2/NOx, and hepatorenal functional deficits by the Tridax procumbens extract.Biomed Pharmacother. 2023 Feb;158:114114. doi: 10.1016/j.biopha.2022.114114. Epub 2022 Dec 14. Biomed Pharmacother. 2023. PMID: 36525818
-
Mechanism of antidiabetic effects of Plicosepalus Acaciae flower in streptozotocin-induced type 2 diabetic rats, as complementary and alternative therapy.BMC Complement Med Ther. 2020 Sep 23;20(1):290. doi: 10.1186/s12906-020-03087-z. BMC Complement Med Ther. 2020. PMID: 32967670 Free PMC article.
-
Dacryodes edulis (G. Don) H.J. Lam modulates glucose metabolism, cholinergic activities and Nrf2 expression, while suppressing oxidative stress and dyslipidemia in diabetic rats.J Ethnopharmacol. 2020 Jun 12;255:112744. doi: 10.1016/j.jep.2020.112744. Epub 2020 Mar 9. J Ethnopharmacol. 2020. PMID: 32165174
-
Herbal Medicines as Complementary Therapy for Managing Complications in COVID-19 Patients with Diabetes Mellitus.Diabetes Metab Syndr Obes. 2025 Jan 16;18:135-146. doi: 10.2147/DMSO.S498774. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 39840393 Free PMC article. Review.
References
-
- Al-Snafi A.E. Pharmacological importance of Clitoria ternatea–a review. IOSR. J. Pharm. 2016;6:68–83.
-
- Atlas D. International diabetes federation. IDF Diabetes Atlas, 7th edn. Brussels, Belgium: Int. Diabetes Federation. 2015;33:1–304.
-
- Balaji B., Bookya K., Bai G.V., Mangilal T. Hepatoprotective agent present in pods of Clitoria ternatea with evidence of histopathological analysis. Int. J. 2015;4:33–38.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
