Generation and characterization of mesenchymal stem cells from the affected femoral heads of dogs with Legg Calvé Perthes disease
- PMID: 38938425
- PMCID: PMC11199743
- DOI: 10.5455/OVJ.2024.v14.i5.12
Generation and characterization of mesenchymal stem cells from the affected femoral heads of dogs with Legg Calvé Perthes disease
Abstract
Background: Canine Legg Calvé Perthes disease (LCPD) occurs during the growth period, and the cause of ischemic necrosis of the femoral head during growth remains unclear. If LCPD-affected femoral head-derived mesenchymal stem cells (LCPD-MSCs) can be generated, they can be used as a new tool for the pathophysiological analysis of canine LCPD.
Aim: To generate affected femoral head-derived mesenchymal stem cells (MSCs) from dogs with LCPD and investigate the mRNA expression levels of angiogenesis-related factors and osteogenic differentiation potency of LCPD-MSCs.
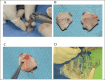
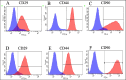
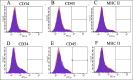
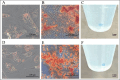
Methods: This study was performed using affected femoral heads from dogs diagnosed with LCPD and underwent femoral head and neck ostectomy. The necrotic tissue was harvested from the LCPD-affected femoral head and cultured statically (LCPD group, n = 6). Canine bone marrow-derived MSCs (BM-MSCs) were used as controls (control group, n = 6). First, the morphology of the cultured cells was observed, and the expression of CD29, CD34, CD44, CD45, CD90, and major histocompatibility complex class II was analyzed using flow cytometry. Additionally, the trilineage differentiation potency of the LCPD-affected head-derived adherent cells was examined. Furthermore, the expression levels of HIF1A, VEGFA, VEGFB, and PDGFB mRNAs and the bone differentiation potency of LCPD-affected head-derived adherent cells were investigated.
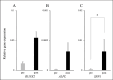
Results: LCPD-affected femoral head-derived adherent cells showed a fibroblast-like morphology, and the expression of cell surface antigens was similar to that of BM-MSCs. In addition, LCPD-affected femoral head-derived adherent cells showed the same trilineage differentiation potency as BM-MSCs and were consistent with MSC characteristics. Furthermore, the mRNA expression levels of angiogenesis-related factors could be objectively measured in LCPD-MSCs and those MSCs had bone differentiation potency.
Conclusion: In the present study, canine LCPD-MSCs were successfully generated, suggesting their usefulness as a tool for pathological analysis of LCPD in dogs.
Keywords: Angiogenesis related gene; Bone differentiation potency; Dog; Legg Calvé Perthes disease; Mesenchymal stem cell.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Damage associated molecular patterns in necrotic femoral head inhibit osteogenesis and promote fibrogenesis of mesenchymal stem cells.Bone. 2022 Jan;154:116215. doi: 10.1016/j.bone.2021.116215. Epub 2021 Sep 24. Bone. 2022. PMID: 34571205 Free PMC article.
-
3D MRI Quantification of Femoral Head Deformity in Legg-Calvé-Perthes Disease.J Orthop Res. 2017 Sep;35(9):2051-2058. doi: 10.1002/jor.23484. Epub 2016 Dec 14. J Orthop Res. 2017. PMID: 27864891
-
Comparative structural analysis of the canine femoral head in Legg-Calvé-Perthes disease.Vet Radiol Ultrasound. 2009 Jul-Aug;50(4):404-11. doi: 10.1111/j.1740-8261.2009.01557.x. Vet Radiol Ultrasound. 2009. PMID: 19697606
-
Legg-Calvé-Perthes disease overview.Orphanet J Rare Dis. 2022 Mar 15;17(1):125. doi: 10.1186/s13023-022-02275-z. Orphanet J Rare Dis. 2022. PMID: 35292045 Free PMC article. Review.
-
Legg Calvé Perthes disease in the dog.Morphologie. 2021 Jun;105(349):143-147. doi: 10.1016/j.morpho.2020.11.011. Epub 2020 Dec 26. Morphologie. 2021. PMID: 33376048 Review.
References
-
- Aguado E., Goyenvalle E. Legg Calvé Perthes disease in the dog. Morphologie. 2021;105:143–147. - PubMed
-
- Bassett F.H.I., Wilson J.W., Allen B.L.J., Azuma H. Normal vascular anatomy of the head of the femur in puppies with emphasis on the inferior retinacular vessels. J. Bone Joint Surg. 1969;51(6):1139–1153. - PubMed
-
- Csaki C., Matis U., Mobasheri A., Ye H., Shakibaei M. Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem. Cell Biol. 2007;128(6):507–520. - PubMed
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D.J., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
