TRIM21 and Fc-engineered antibodies: decoding its complex antibody binding mode with implications for viral neutralization
- PMID: 38938560
- PMCID: PMC11210195
- DOI: 10.3389/fimmu.2024.1401471
TRIM21 and Fc-engineered antibodies: decoding its complex antibody binding mode with implications for viral neutralization
Abstract
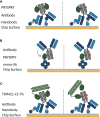
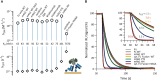
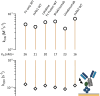
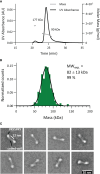
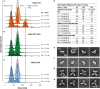
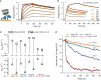
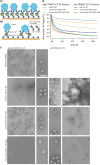
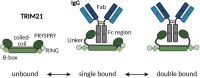
TRIM21 is a pivotal effector in the immune system, orchestrating antibody-mediated responses and modulating immune signaling. In this comprehensive study, we focus on the interaction of TRIM21 with Fc engineered antibodies and subsequent implications for viral neutralization. Through a series of analytical techniques, including biosensor assays, mass photometry, and electron microscopy, along with structure predictions, we unravel the intricate mechanisms governing the interplay between TRIM21 and antibodies. Our investigations reveal that the TRIM21 capacity to recognize, bind, and facilitate the proteasomal degradation of antibody-coated viruses is critically dependent on the affinity and avidity interplay of its interactions with antibody Fc regions. We suggest a novel binding mechanism, where TRIM21 binding to one Fc site results in the detachment of PRYSPRY from the coiled-coil domain, enhancing mobility due to its flexible linker, thereby facilitating the engagement of the second site, resulting in avidity due to bivalent engagement. These findings shed light on the dual role of TRIM21 in antiviral immunity, both in recognizing and directing viruses for intracellular degradation, and demonstrate its potential for therapeutic exploitation. The study advances our understanding of intracellular immune responses and opens new avenues for the development of antiviral strategies and innovation in tailored effector functions designed to leverage TRIM21s unique binding mode.
Keywords: TRIM21; affinity; antibody Fc mutants; antibody mediated viral neutralization; avidity; binding kinetics; structure-function; therapeutic IgG.
Copyright © 2024 Reusch, Franken, Then, Ringler, Butzer, Juroschek, Klein, Schlothauer and Larivière.
Conflict of interest statement
CK declares employment, patents and stock ownership with Roche. Authors JR, JT, JB, TJ, TS, and LL were employed by the company Roche Diagnostics GmbH. Authors LF and PR were employed by the company F.Hoffmann-La Roche Ltd. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

