Disease-specific autoantibody production in the lungs and salivary glands of anti-synthetase syndrome
- PMID: 38938569
- PMCID: PMC11208671
- DOI: 10.3389/fimmu.2024.1265792
Disease-specific autoantibody production in the lungs and salivary glands of anti-synthetase syndrome
Abstract
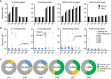
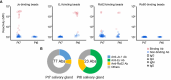
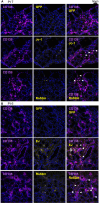
Interstitial lung disease is a common complication of anti-synthetase syndrome (ASS), and lymphocytic infiltration is often observed in the lesion. We have recently reported that disease-specific autoantibodies are produced by infiltrating lymphocytes in some autoimmune diseases. Here, we investigate the antigen specificity of B cells in the lung lesions of ASS patients. A total of 177 antibodies were produced from antibody-secreting cells in bronchoalveolar fluid (BALF) of three each of serum anti-Jo-1 and serum anti-EJ antibody-positive patients. Twelve to 30% and 50 to 62% of these antibodies were disease-specific autoantibodies, respectively. These autoantibodies recognized conformational epitopes of the whole self-antigen and had affinity maturations, indicating that self-antigens themselves are the target of humoral immunity. In addition, 100 antibodies were produced from two salivary gland tissues, obtained by chance, of ASS patients. Salivary glands are not generally recognized as lesions of ASS, but unexpectedly, ASS-related autoantibody production was also observed similar to that of BALF. Immunostaining confirmed the presence of ASS-related autoantibody-producing cells in salivary glands. Our results suggest that disease-specific autoantibody production at lesion sites is a common pathogenesis of autoimmune diseases, and that tissue-specific production of autoantibodies can provide insights regarding the distribution of organ manifestations in autoimmune diseases.
Keywords: anti-Ro52 antibody; anti-aminoacyl-tRNA synthetase antibody; anti-synthetase syndrome; autoantibody; idiopathic inflammatory myopathy; salivary gland.
Copyright © 2024 Takeshita, Suzuki, Nakazawa, Kamata, Ishii, Oyamada, Oshima, Usuda, Tsunoda and Takeuchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

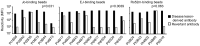

References
-
- Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, et al. . Anti-aminoacyl-trna synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. (2006) 39:233–41. doi: 10.1080/08916930600622884 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

