Distinct targeting and uptake of platelet and red blood cell-derived extracellular vesicles into immune cells
- PMID: 38938679
- PMCID: PMC11080822
- DOI: 10.1002/jex2.130
Distinct targeting and uptake of platelet and red blood cell-derived extracellular vesicles into immune cells
Abstract
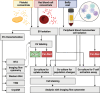
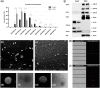
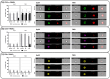
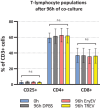
Blood-derived extracellular vesicles (EVs) hold great therapeutic potential. As blood contains mixed EV populations, it is challenging to study EVs originating from different cells separately. Blood cell concentrates manufactured in blood banks offer an excellent non-invasive source of blood cell-specific EV populations. To study blood cell-specific EVs, we isolated EVs from platelet (TREVs) and red blood cell (EryEVs) concentrates and characterized them using nanoparticle tracking analysis, imaging flow cytometry, electron microscopy and western blot analysis and co-cultured them with peripheral blood mononuclear cells (PBMCs). Our aim was to use imaging flow cytometry to investigate EV interaction with PBMCs as well as study their effects on T-lymphocyte populations to better understand their possible biological functions. As a conclusion, TREVs interacted with PBMCs more than EryEVs. Distinctively, TREVs were uptaken into CD11c+ monocytes rapidly and into CD19+ B-lymphocytes in 24 h. EryEVs were not uptaken into CD11c+ monocytes before the 24-h time point, and they were only seen on the surface of lymphocytes. Neither TREVs nor EryEV were uptaken into CD3+ T-lymphocytes and no effect on T-cell populations was detected. We have previously seen similar differences in targeting PC-3 cancer cells. Further studies are needed to address the functional properties of blood cell concentrate-derived EVs. This study demonstrates that imaging flow cytometry can be used to study the distinctive differences in the interaction and uptake of EVs. Considering our current and previous results, EVs present a new valuable component for the future development of blood-derived therapeutics.
Keywords: blood cell; extracellular vesicle; imaging flow cytometry; platelet; red.
© 2024 Finnish Red Cross Blood Service. Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12(5), 614–627. 10.1111/jth.12554 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
