The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia
- PMID: 38938723
- PMCID: PMC11198175
- DOI: 10.1016/j.jacadv.2023.100648
The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia
Abstract
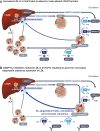
Background: Homozygous familial hypercholesterolemia (HoFH) is characterized by early-onset atherosclerotic cardiovascular disease due to the high low-density lipoprotein cholesterol (LDL-C) burden. Patients with null-null low-density lipoprotein receptor (LDLR) variants respond poorly, if at all, to statins and proprotein convertase subtilisin/kexin type 9 inhibitors, which act by upregulating LDLR expression. The 24-week double-blind treatment period (DBTP) of the phase 3 ELIPSE HoFH (Evinacumab Lipid Studies in Patients with Homozygous Familial hypercholesterolemia; NCT03399786) study demonstrated significant LDL-C reductions in patients with HoFH; LDL-C reductions were also observed in those with null-null LDLR mutations.
Objectives: The purpose of this study was to evaluate longer-term efficacy and safety of evinacumab in patients with HoFH from the ELIPSE HoFH study.
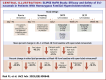
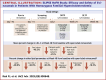
Methods: Patients with HoFH on stable lipid-lowering therapies (LLTs) ± lipoprotein apheresis and screening LDL-C ≥70 mg/dL who completed the DBTP entered the 24-week open-label treatment period (OLTP) and received intravenous evinacumab 15 mg/kg every 4 weeks. OLTP results were summarized descriptively.
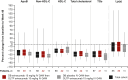
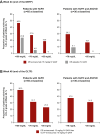
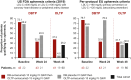
Results: A total of 64 patients completed the DBTP and received open-label evinacumab. Despite multiple LLTs, the mean baseline LDL-C at DBTP entry was 250.5 ± 162.3 mg/dL. From baseline to week 48 (end of OLTP), evinacumab reduced mean LDL-C by 46.3% (mean reduction, 134.3 ± 117.3 mg/dL), with similar mean LDL-C reductions for patients with null-null (47.2%) and non-null variants (45.9%). Adverse events occurred in 47 (73.4%) patients; 4 (6.3%) patients experienced adverse events considered evinacumab-related (drug hypersensitivity, infusion-related reaction and asthenia, generalized pruritis, and muscle spasms).
Conclusions: In patients with HoFH, evinacumab demonstrated substantial and sustained LDL-C reduction regardless of LDLR function, and was generally well tolerated.
Keywords: angiopoietin-like protein 3; clinical trial; evinacumab; homozygous familial hypercholesterolemia; lipids; lipoprotein.
© 2023 The Authors.
Conflict of interest statement
This study was funded by 10.13039/100009857Regeneron Pharmaceuticals, Inc. Dr Raal reports grants from Regeneron Pharmaceuticals, Inc, during the conduct of the study, and personal fees from Amgen, Sanofi-Aventis, Regeneron Pharmaceuticals, Inc, Novartis, and LIB Therapeutics outside the submitted work. Dr Rosenson has received research grants from Amgen, Arrowhead, Lilly, Novartis, and Regeneron Pharmaceuticals, Inc; has received consulting honoraria from Amgen, CVS Caremark, Novartis, and Regeneron Pharmaceuticals, Inc; has received honoraria from Amgen, Kowa, Lilly, and Regeneron Pharmaceuticals, Inc; has received royalties from Wolters Kluwer (UpToDate); and has stock ownership in MediMergent, LLC. Dr Reeskamp has received investigational fees from Regeneron Pharmaceuticals, Inc during the conduct of the study; and is a co-founder of Lipid Tools B.V. Dr Kastelein has received personal fees from AstraZeneca, CIVI Biotechnology, CSL Behring, Draupnir, Esperion Therapeutics, Inversago, Madrigal Pharmaceuticals, Menarini, North Sea Therapeutics, Novo Nordisk, Novartis, RegenXBio, Sirnaomics, Regeneron Pharmaceuticals, Inc, Scribe Therapeutics, Staten Biotech, 89 Bio, and Omeicos outside the submitted work; and ownership in New Amsterdam Pharma. Dr Rubba has received grants from Regeneron Pharmaceuticals, Inc during the conduct of the study and outside the submitted work. Dr Duell has received research grants and/or consultancy fees from Akcea/Ionis, Esperion Therapeutics, Kaneka, Novo Nordisk, Regeneron Pharmaceuticals, Inc, RegenXBio, and Retrophin/Travere. Dr Koseki has received research grants from Kowa. Dr Stroes has received grants and/or personal fees from Amgen, Akcea, Athera, Esperion Therapeutics, Mylan, Novartis, Regeneron Pharmaceuticals, Inc, and Sanofi. Drs Ali, Banerjee, Chan, Khilla, McGinniss, Pordy, and Zhang are employees and/or shareholders of Regeneron Pharmaceuticals, Inc. Dr Gaudet has received grants and personal fees from Regeneron Pharmaceuticals, Inc during the conduct of the study; and grants and/or personal fees from Aegerion, Amgen, Amryt Pharma, Arrowhead, Eli Lilly, Esperion Therapeutics, Pfizer, Sanofi, The Medicines Company, and Verve Therapeutics outside the submitted work.
Figures

References
-
- Cuchel M., Bruckert E., Ginsberg H.N., et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–2157. - PMC - PubMed
-
- de Ferranti S.D., Rodday A.M., Mendelson M.M., et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES) Circulation. 2016;133:1067–1072. - PubMed
-
- France M., Rees A., Datta D., et al. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016;255:128–139. - PubMed
-
- Bruckert E. Recommendations for the management of patients with homozygous familial hypercholesterolaemia: overview of a new European Atherosclerosis Society Consensus Statement. Atheroscler Suppl. 2014;15:26–32. - PubMed
LinkOut - more resources
Full Text Sources

