Sex Differences in Cardiovascular Outcomes and Cholesterol-Lowering Efficacy of PCSK9 Inhibitors: Systematic Review and Meta-Analysis
- PMID: 38938736
- PMCID: PMC11198239
- DOI: 10.1016/j.jacadv.2023.100669
Sex Differences in Cardiovascular Outcomes and Cholesterol-Lowering Efficacy of PCSK9 Inhibitors: Systematic Review and Meta-Analysis
Abstract
Background: Guideline-recommended low-density lipoprotein cholesterol (LDL-C) thresholds are often not achieved in women. The proprotein convertase subtilisin/kexin type-9 inhibitor (PCSK9i) monoclonal antibodies can help further reduce LDL-C and major adverse cardiovascular events (MACE) although differences in efficacy by sex and type are less understood.
Objectives: The authors sought to determine if there are differences in the efficacy of LDL-C lowering and reduction in the risk of MACE by sex and type of PCSK9i.
Methods: A comprehensive literature search was done through October 17, 2022, for published trials comparing PCSK9i vs control. Outcomes assessed were LDL-C reduction and incidence of MACE following the use of PCSK9i vs placebo, stratified by sex and type of PCSK9i used.
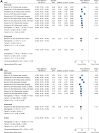
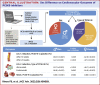
Results: We identified 16 trials with 54,996 adults, and 15,143 (27.5%) of them were female. PCSK9i significantly reduced MACE compared to placebo in both women (HR: 0.86, 95% CI: 0.74-0.97, P < 0.001) and men (HR: 0.85, 95% CI: 0.79-0.91, P < 0.001) with no significant sex difference (MD -0.01, 95% CI: -0.14 to -0.13, P = 0.930). PCSK9i also significantly reduced LDL-C levels in both sexes at 12 weeks (females: MD -62.57, 95% CI: -70.24 to -54.91, P < 0.001; males: MD -66.19, 95% CI: -72.03 to -60.34, P < 0.001) and 24 weeks (females: MD -47.52, 95% CI: -52.94 to -42.09, P < 0.001; males: MD -54.07, 95% CI: -59.46 to -48.68, P < 0.001). Significant sex difference was seen in the LDL reduction of PCSK9i for both 12 weeks (males vs females: MD -4.55, 95% CI: -7.34 to -1.75, P < 0.01) and 24 weeks (males vs females: MD -7.11, 95% CI: -9.99 to -4.23, P < 0.001).
Conclusions: The use of PCSK9i results in significant LDL-C and MACE reduction in both males and females. While there is no significant sex difference in MACE reduction, LDL-C reduction is greater in males than in females. Our data support the equal use of PCSK9i in all eligible patients, regardless of sex.
Keywords: PCSK9 inhibitors; cardiovascular events; sex difference; tertiary prevention.
© 2023 The Authors.
Conflict of interest statement
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






References
-
- Atar D., Jukema J.W., Molemans B., et al. New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis. 2021;319:51–61. - PubMed
-
- Dixon D.L., Pamulapati L.G., Bucheit J.D., et al. Recent updates on the use of PCSK9 inhibitors in patients with atherosclerotic cardiovascular disease. Curr Atheroscler Rep. 2019;21(5):16. - PubMed
-
- Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366–1418. - PubMed
-
- Khan S.U., Yedlapati S.H., Lone A.N., et al. PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction: a systematic review and network meta-analysis. BMJ. 2022;377 - PubMed
-
- Fulcher J., O'Connell R., Voysey M., et al. Efficacy and safety of LDL-lowering therapy among males and females: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

