Extracellular Vesicle Spherical Nucleic Acids
- PMID: 38938802
- PMCID: PMC11200237
- DOI: 10.1021/jacsau.4c00338
Extracellular Vesicle Spherical Nucleic Acids
Abstract
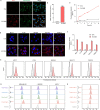
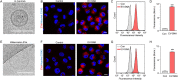
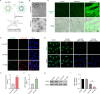
Extracellular vesicles (EVs) are naturally occurring vesicles secreted by cells that can transport cargo between cells, making them promising bioactive nanomaterials. However, due to the complex and heterogeneous biological characteristics, a method for robust EV manipulation and efficient EV delivery is still lacking. Here, we developed a novel class of extracellular vesicle spherical nucleic acid (EV-SNA) nanostructures with scalability, programmability, and efficient cellular delivery. EV-SNA was constructed through the simple hydrophobic coassembly of natural EVs with cholesterol-modified oligonucleotides and can be stable for 1 month at room temperature. Based on programmable nucleic acid shells, EV-SNA can respond to AND logic gates to achieve vesicle assembly manipulation. Importantly, EV-SNA can be constructed from a wide range of biological sources EV, enhancing cellular delivery capability by nearly 10-20 times. Compared to artificial liposomal SNA, endogenous EV-SNA exhibited better biocompatibility and more effective delivery of antisense oligonucleotides in hard-to-transfect primary stem cells. Additionally, EV-SNA can deliver functional EVs for immune regulation. As a novel material form, EV-SNA may provide a modular and programmable framework paradigm for EV-based applications in drug delivery, disease treatment, nanovaccines, and other fields.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- You Y.; Tian Y.; Yang Z.; Shi J.; Kwak K. J.; Tong Y.; Estania A. P.; Cao J.; Hsu W. H.; Liu Y.; Chiang C. L.; Schrank B. R.; Huntoon K.; Lee D.; Li Z.; Zhao Y.; Zhang H.; Gallup T. D.; Ha J.; Dong S.; Li X.; Wang Y.; Lu W. J.; Bahrani E.; Lee L. J.; Teng L.; Jiang W.; Lan F.; Kim B. Y. S.; Lee A. S. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 2023, 7 (7), 887–900. 10.1038/s41551-022-00989-w. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
