Cancer cell invasion alters the protein profile of extracellular vesicles
- PMID: 38938900
- PMCID: PMC11080925
- DOI: 10.1002/jex2.124
Cancer cell invasion alters the protein profile of extracellular vesicles
Abstract
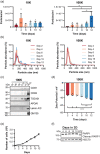
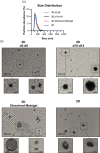
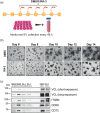
Extracellular vesicles (EVs) are important mediators of intercellular communication involved in local and long-range signalling of cancer metastasis. The onset of invasion is the key step of the metastatic cascade, but the secretion of EVs has remained unexplored at that stage due to technical challenges. In this study, we present a platform to track EVs over the course of invasive development of human prostate cancer cell (PC3) tumoroids utilizing in vivo-mimicking extracellular matrix-based 3D cultures. Using this EV production method, combined with proteomic profiling, we show that PC3 tumoroids secrete EVs with previously undefined protein cargo. Intriguingly, an increase in EV amounts and extensive changes in the EV protein composition were detected upon invasive transition of the tumoroids. The changes in EV protein cargo were counteracted by chemical inhibition of invasion. These results reveal the impact of the tumoroids' invasive status on EV secretion and cargo, and highlight the necessity of in vivo-mimicking conditions for uncovering novel cancer-derived EV components.
Keywords: 3D cultures; EV heterogeneity; EV proteome; PC3 cells; extracellular vesicles; invasion; prostate cancer.
© 2023 The Authors. Journal of Extracellular Biology published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Álvez, M. B. , Edfors, F. , von Feilitzen, K. , Zwahlen, M. , Mardinoglu, A. , Edqvist, P. H. , Sjöblom, T. , Lundin, E. , Rameika, N. , Enblad, G. , Lindman, H. , Höglund, M. , Hesselager, G. , Stålberg, K. , Enblad, M. , Simonson, O. E. , Häggman, M. , Axelsson, T. , Åberg, M. , … Uhlén, M. (2023). Next generation pan‐cancer blood proteome profiling using proximity extension assay. Nature Communications, 14(1), 4308. 10.1038/s41467-023-39765-y - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
