A Transcriptomic Biomarker Predicting Linezolid-Associated Neuropathy During Treatment of Drug-Resistant Tuberculosis
- PMID: 38939039
- PMCID: PMC11210591
- DOI: 10.20411/pai.v9i2.705
A Transcriptomic Biomarker Predicting Linezolid-Associated Neuropathy During Treatment of Drug-Resistant Tuberculosis
Abstract
Background: Neuropathic adverse events occur frequently in linezolid-containing regimens, some of which remain irreversible after drug discontinuation.
Objective: We aimed to identify and validate a host RNA-based biomarker that can predict linezolid-associated neuropathy before multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB) treatment initiation and to identify genes and pathways that are associated with linezolid-associated neuropathy.
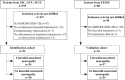
Methods: Adult patients initiating MDR/RR-TB treatment including linezolid were prospectively enrolled in 3 independent cohorts in Germany. Clinical data and whole blood RNA for transcriptomic analysis were collected. The primary outcome was linezolid-associated optic and/or peripheral neuropathy. A random forest algorithm was used for biomarker identification. The biomarker was validated in an additional fourth cohort of patients with MDR/RR-TB from Romania.
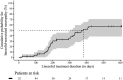
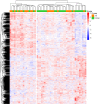
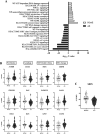
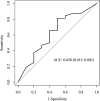
Results: A total of 52 patients from the 3 identification cohorts received linezolid treatment. Of those, 24 (46.2%) developed peripheral and/or optic neuropathies during linezolid treatment. The majority (59.3%) of the episodes were of moderate (grade 2) severity. In total, the expression of 1,479 genes differed significantly at baseline of treatment. Suprabasin (SBSN) was identified as a potential biomarker to predict linezolid-associated neuropathy. In the validation cohort, 10 of 42 (23.8%) patients developed grade ≥3 neuropathies. The area under the curve for the biomarker algorithm prediction of grade ≥3 neuropathies was 0.63 (poor; 95% confidence interval: 0.42 - 0.84).
Conclusions: We identified and preliminarily validated a potential clinical biomarker to predict linezolid-associated neuropathies before the initiation of MDR/RR-TB therapy. Larger studies of the SBSN biomarker in more diverse populations are warranted.
Keywords: SBSN; adverse events; linezolid; multidrug resistance; neurotoxicity; precision medicine; tuberculosis.
Copyright © 2024 Pathogens and Immunity.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- World Health Organization. Global tuberculosis report 2023. Geneva: World Health Organization; 2023.
-
- World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment. 2022. update ed. Geneva: World Health Organization; 2022. - PubMed
-
- Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Nix TBTT. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. PubMed PMID: 32130813; PMCID: PMC6955640. - DOI - PMC - PubMed
-
- Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, Brust JCM, Campbell JR, Chang VWL, Falzon D, Guglielmetti L, Isaakidis P, Kempker RR, Kipiani M, Kuksa L, Lange C, Laniado-Laborin R, Nahid P, Rodrigues D, Singla R, Udwadia ZF, Menzies D, Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–94. doi: 10.1016/S2213-2600(20)30047-3. PubMed PMID: 32192585; PMCID: PMC7384398. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
