The slow photo-induced CO2 release of N-phthaloylglycine
- PMID: 38939141
- PMCID: PMC11206210
- DOI: 10.1039/d4sc01604a
The slow photo-induced CO2 release of N-phthaloylglycine
Abstract
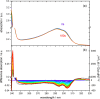
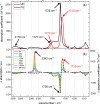
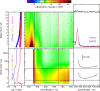
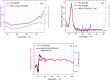
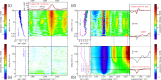
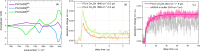
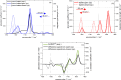
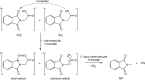
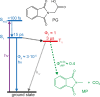
Carboxylic acids and carboxylates may release CO2 upon oxidation. The oxidation can be conducted electrochemically as in the Kolbe synthesis or by a suitable oxidant. In N-phthaloylglycine (PG), the photo-excited phthalimide chromophore acts as an oxidant. Here, the photo-kinetics of PG dissolved in acetonitrile is traced by steady-state as well as time-resolved UV/vis and IR spectroscopy. The experiments provide clear evidence that, contrary to earlier claims, the photo-induced CO2 release is slow, i.e. it occurs on the microsecond time range. The triplet state of PG is, therefore, the photo-reactive one.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
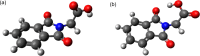
References
-
- Schäfer H. J., Recent contributions of Kolbe electrolysis to organic synthesis, Springer Berlin Heidelberg, Berlin, Heidelberg, 1990
-
- Palkovits S. Palkovits R. Chem. Ing. Tech. 2019;91:699–706. doi: 10.1002/cite.201800205. - DOI
-
- Sorigue D. Hadjidemetriou K. Blangy S. Gotthard G. Bonvalet A. Coquelle N. Samire P. Aleksandrov A. Antonucci L. Benachir A. Boutet S. Byrdin M. Cammarata M. Carbajo S. Cuine S. Doak R. B. Foucar L. Gorel A. Grunbein M. Hartmann E. Hienerwadel R. Hilpert M. Kloos M. Lane T. J. Legeret B. Legrand P. Li-Beisson Y. Moulin S. L. Y. Nurizzo D. Peltier G. Schiro G. Shoeman R. L. Sliwa M. Solinas X. Zhuang B. Barends T. R. M. Colletier J. P. Joffre M. Royant A. Berthomieu C. Weik M. Domratcheva T. Brettel K. Vos M. H. Schlichting I. Arnoux P. Muller P. Beisson F. Science. 2021;372:eabd5687. doi: 10.1126/science.abd5687. - DOI - PubMed
LinkOut - more resources
Full Text Sources

