Cardio-Oncology Recommendations for Pediatric Oncology Patients: An Australian and New Zealand Delphi Consensus
- PMID: 38939459
- PMCID: PMC11198111
- DOI: 10.1016/j.jacadv.2022.100155
Cardio-Oncology Recommendations for Pediatric Oncology Patients: An Australian and New Zealand Delphi Consensus
Abstract
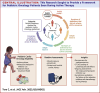
Cardio-oncology is a new multidisciplinary area of expertise that seeks to pre-emptively and proactively address cardiac complications that emerge during and following cancer therapy. Modern therapies including molecular targeted therapy and immunotherapy have broadened the agents that can cause cardiac sequelae, often with complications arising within days to weeks of therapy. Several international guidelines have been developed for the acute monitoring of cardio-oncology side effects. However, none are specific to pediatrics. We have addressed this gap in the literature by undertaking a rigorous Delphi consensus approach across 11 domains of cardio-oncology care using an Australian and New Zealand expert group. The expert group consisted of pediatric and adult cardiologists and pediatric oncologists. This Delphi consensus provides an approach to perform risk and baseline assessment, screening, and follow-up, specific to the cancer therapeutic. This review is a useful tool for clinicians involved in the cardio-oncology care of pediatric oncology patients.
Keywords: acute cardio-oncology; anthracyclines; cardio-oncology; checkpoint inhibitors; frameworks for cardio-oncology; models of care; molecular inhibitors; pediatric oncology; radiation therapy.
© 2022 The Authors. Published by Elsevier on behalf of the American College of Cardiology Foundation.
Conflict of interest statement
This study was funded by the 10.13039/100002129Heart Foundation as part of the Strategic Cardio-Oncology Grants (105525). The funder of the study had no role in the study design, data collection, data analysis, data interpretation or the writing of this report. Dr Toro is supported by Children’s Cancer Foundation (CCF). Dr Conyers is supported by the Kids Cancer Project, The Royal Children’s Hospital Foundation, Heart Foundation of Australia, and Victorian Paediatric Cancer Consortium and holds a Murdoch Children’s Research Institute (MCRI) Clinician Scientist Fellowship. Dr Elliott is a member of the Novo Nordisk Foundation Center for Stem Cell Medicine; is supported by a Novo Nordisk Foundation grant number NNF21CC0073729; and is supported by the National Health and Medical Research Council of Australia, Heart Foundation of Australia, and The Medical Research Future Fund. Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. - PubMed
-
- Franco V.I., Lipshultz S.E. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young. 2015;25 Suppl 2:107–116. - PubMed
-
- Conyers R., Costello B., La Gerche A., et al. Chemotherapy-related cardiotoxicity: are Australian practitioners missing the point? Intern Med J. 2017;47(10):1166–1172. - PubMed
-
- Zamorano J.L., Lancellotti P., Rodriguez Munoz D., et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. - PubMed
-
- Tukenova M., Guibout C., Oberlin O., et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308–1315. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

