Guidelines for the purification and characterization of extracellular vesicles of parasites
- PMID: 38939734
- PMCID: PMC11080789
- DOI: 10.1002/jex2.117
Guidelines for the purification and characterization of extracellular vesicles of parasites
Abstract
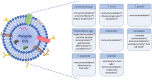
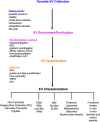
Parasites are responsible for the most neglected tropical diseases, affecting over a billion people worldwide (WHO, 2015) and accounting for billions of cases a year and responsible for several millions of deaths. Research on extracellular vesicles (EVs) has increased in recent years and demonstrated that EVs shed by pathogenic parasites interact with host cells playing an important role in the parasite's survival, such as facilitation of infection, immunomodulation, parasite adaptation to the host environment and the transfer of drug resistance factors. Thus, EVs released by parasites mediate parasite-parasite and parasite-host intercellular communication. In addition, they are being explored as biomarkers of asymptomatic infections and disease prognosis after drug treatment. However, most current protocols used for the isolation, size determination, quantification and characterization of molecular cargo of EVs lack greater rigor, standardization, and adequate quality controls to certify the enrichment or purity of the ensuing bioproducts. We are now initiating major guidelines based on the evolution of collective knowledge in recent years. The main points covered in this position paper are methods for the isolation and molecular characterization of EVs obtained from parasite-infected cell cultures, experimental animals, and patients. The guideline also includes a discussion of suggested protocols and functional assays in host cells.
Keywords: EVs methodology; extracellular vesicles; helminth; host‐parasite interaction; infection; protocols; protozoan parasites.
© 2023 The Authors. Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
No potential conflicts of interest were reported by the authors.
Figures
References
-
- Abdi, A. I. , Hodgson, S. H. , Muthui, M. K. , Kivisi, C. A. , Kamuyu, G. , Kimani, D. , Hoffman, S. L. , Juma, E. , Ogutu, B. , Draper, S. J. , Osier, F. , Bejon, P. , Marsh, K. , & Bull, P. C. (2017). Plasmodium falciparum malaria parasite var gene expression is modified by host antibodies: Longitudinal evidence from controlled infections of Kenyan adults with varying natural exposure. BMC Infectious Diseases [Electronic Resource], 17, 585. - PMC - PubMed
-
- Abdossamadi, Z. , Taheri, T. , Seyed, N. , Montakhab‐Yeganeh, H. , Zahedifard, F. , Taslimi, Y. , Habibzadeh, S. , Gholami, E. , Gharibzadeh, S. , & Rafati, S. (2017). Live Leishmania tarentolae secreting HNP1 as an immunotherapeutic tool against Leishmania infection in BALB/c mice. Immunotherapy, 9, 1089–1102. - PubMed
-
- Abou Karam, P. , Rosenhek‐Goldian, I. , Ziv, T. , Ben Ami Pilo, H. , Azuri, I. , Rivkin, A. , Kiper, E. , Rotkopf, R. , Cohen, S. R. , Torrecilhas, A. C. , Avinoam, O. , Rojas, A. , Morandi, M. I. , & Regev‐Rudzki, N. (2022). Malaria parasites release vesicle subpopulations with signatures of different destinations. Embo Reports, 23, e54755. - PMC - PubMed
-
- Abuin, G. , Colli, W. , & Alves, M. J. (1996a). Turnover and shedding of the Tc‐85 surface glycoprotein of Trypanosoma cruzi trypomastigotes. Brazilian Journal of Medical and Biological Research, 29, 335–341. - PubMed
-
- Abuin, G. , Colli, W. , de Souza, W. , & Alves, M. J. (1989). A surface antigen of Trypanosoma cruzi involved in cell invasion (Tc‐85) is heterogeneous in expression and molecular constitution. Molecular and Biochemical Parasitology, 35, 229–237. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
