ISG15 mediates the function of extracellular vesicles in promoting ovarian cancer progression and metastasis
- PMID: 38939897
- PMCID: PMC11080709
- DOI: 10.1002/jex2.92
ISG15 mediates the function of extracellular vesicles in promoting ovarian cancer progression and metastasis
Abstract
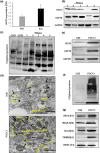
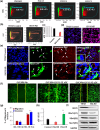
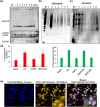
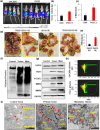
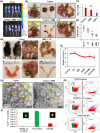
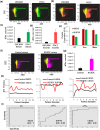
The interferon stimulated gene 15 (ISG15), a ubiquitin like protein and its conjugates have been implicated in various human malignancies. However, its role in ovarian cancer progression and metastasis is largely unknown. In high grade serous ovarian cancer (HGSOC), ascites is the major contributor to peritoneal metastasis. In this study, we identified significantly elevated ISG15 protein expression in HGSOC patient ascites, ascites derived primary ovarian cancer cells (POCCs), POCC small extracellular vesicles (sEVs) as well as metastatic tissue. Our results demonstrates that ISG15 increases exocytosis in ascites-derived POCCs by decreasing the endosome-lysosomal fusion, indicating a key role in sEV secretion. Further, knockdown (KD) of ISG15 resulted in a significant decrease in vesicles secretion from HGSOC cells and in vivo mouse models, leading to reduced HGSOC cell migration and invasion. Furthermore, our pre-clinical mouse model studies revealed the influence of vesicular ISG15 on disease progression and metastasis. In addition, knockdown of ISG15 or using the ISG15 inhibitor, DAP5, in combination therapy with carboplatin showed to improve the platinum sensitivity in-vitro and reduce tumour burden in-vivo. We also found that ISG15 expression within sEV represents a promising prognostic marker for HGSOC patients. Our findings suggest that ISG15 is a potential therapeutic target for inhibiting progression and metastasis in HGSOC and that vesicular ISG15 expression could be a promising biomarker in the clinical management of ovarian cancer. Significance: High-grade serous ovarian cancer (HGSOC) has high morbidity and mortality rates, but its progression and metastasis are still poorly understood, and there is an urgent need for early detection and targeted therapies. Our study presents novel findings that implicate ISG15-mediated vesicular proteins in the advancement and spread of HGSOC. These results offer pre-clinical evidence of potential new molecular targets, prognostic markers and therapeutic strategies for HGSOC that could ultimately enhance patient survival.
Keywords: ISG15; ISGylation; ascites; extracellular vesicles; metastasis and biomarker; ovarian cancer.
© 2024 The Authors. Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures
Similar articles
-
ISG15 Promotes ERK1 ISGylation, CD8+ T Cell Activation and Suppresses Ovarian Cancer Progression.Cancers (Basel). 2018 Nov 22;10(12):464. doi: 10.3390/cancers10120464. Cancers (Basel). 2018. PMID: 30469497 Free PMC article.
-
Tumor Small Extracellular Vesicle-Transmitted LncRNA CATED Promotes Platinum-Resistance in High-Grade Serous Ovarian Cancer.Adv Sci (Weinh). 2025 Jun 10:e05963. doi: 10.1002/advs.202505963. Online ahead of print. Adv Sci (Weinh). 2025. PMID: 40492382
-
Extracellular vesicles display distinct glycosignatures in high-grade serous ovarian carcinoma.BBA Adv. 2025 Jan 10;7:100140. doi: 10.1016/j.bbadva.2025.100140. eCollection 2025. BBA Adv. 2025. PMID: 39911812 Free PMC article.
-
Extracellular matrix in high-grade serous ovarian cancer: Advances in understanding of carcinogenesis and cancer biology.Matrix Biol. 2023 Apr;118:16-46. doi: 10.1016/j.matbio.2023.02.004. Epub 2023 Feb 11. Matrix Biol. 2023. PMID: 36781087 Review.
-
The high-grade serous ovarian cancer metastasis and chemoresistance in 3D models.Biochim Biophys Acta Rev Cancer. 2024 Jan;1879(1):189052. doi: 10.1016/j.bbcan.2023.189052. Epub 2023 Dec 12. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38097143 Review.
Cited by
-
Obesity-induced extracellular vesicles proteins drive the endometrial cancer pathogenesis: therapeutic potential of HO-3867 and Metformin.Oncogene. 2024 Nov;43(49):3586-3597. doi: 10.1038/s41388-024-03182-2. Epub 2024 Oct 16. Oncogene. 2024. PMID: 39414985 Free PMC article.
References
-
- Alcalá, S. , Sancho, P. , Martinelli, P. , Navarro, D. , Pedrero, C. , Martín‐Hijano, L. , Valle, S. , Earl, J. , Rodríguez‐Serrano, M. , Ruiz‐Cañas, L. , Rojas, K. , Carrato, A. , García‐Bermejo, L. , Fernández‐Moreno, M. Á. , Hermann, P. C. , & Sainz, B. (2020). ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nature Communications, 11(2682), 1–17. 10.1038/s41467-020-16395-2 - DOI - PMC - PubMed
-
- Basters, A. , Geurink, P. P. , Röcker, A. , Witting, K. F. , Tadayon, R. , Hess, S. , Semrau, M. S. , Storici, P. , Ovaa, H. , Knobeloch, K. P. , & Fritz, G. (2017). Structural basis of the specificity of USP18 toward ISG15. Nature Structural & Molecular Biology, 24, 270–278. 10.1038/nsmb.3371 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
