Comparative and integrated analysis of plasma extracellular vesicle isolation methods in healthy volunteers and patients following myocardial infarction
- PMID: 38939906
- PMCID: PMC11080728
- DOI: 10.1002/jex2.66
Comparative and integrated analysis of plasma extracellular vesicle isolation methods in healthy volunteers and patients following myocardial infarction
Abstract
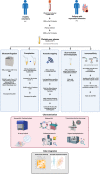
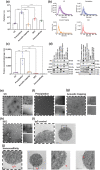
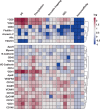
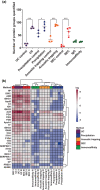
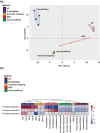
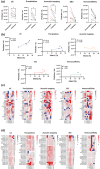
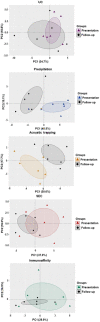
Plasma extracellular vesicle (EV) number and composition are altered following myocardial infarction (MI), but to properly understand the significance of these changes it is essential to appreciate how the different isolation methods affect EV characteristics, proteome and sphingolipidome. Here, we compared plasma EV isolated from platelet-poor plasma from four healthy donors and six MI patients at presentation and 1-month post-MI using ultracentrifugation (UC), polyethylene glycol precipitation, acoustic trapping, size-exclusion chromatography (SEC) and immunoaffinity capture. The isolated EV were evaluated by Nanoparticle Tracking Analysis (NTA), Western blot, transmission electron microscopy (TEM), an EV-protein array, untargeted proteomics (LC-MS/MS) and targeted sphingolipidomics (LC-MS/MS). The application of the five different plasma EV isolation methods in patients presenting with MI showed that the choice of plasma EV isolation method influenced the ability to distinguish elevations in plasma EV concentration following MI, enrichment of EV-cargo (EV-proteins and sphingolipidomics) and associations with the size of the infarct determined by cardiac magnetic resonance imaging 6 months post-MI. Despite the selection bias imposed by each method, a core of EV-associated proteins and lipids was detectable using all approaches. However, this study highlights how each isolation method comes with its own idiosyncrasies and makes the comparison of data acquired by different techniques in clinical studies problematic.
Keywords: acoustic trapping; human; immunoaffinity capture; omics; plasma; precipitation; size exclusion chromatography; ultracentrifugation.
© 2022 The Authors. Journal of Extracellular Biology published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The Author(s) declare that there is no conflict of interest.
Figures

References
-
- Akawi, N. , Checa, A. , Antonopoulos, A. S. , Akoumianakis, I. , Daskalaki, E. , Kotanidis, C. P. , Kondo, H. , Lee, K. , Yesilyurt, D. , Badi, I. , Polkinghorne, M. , Akbar, N. , Lundgren, J. , Chuaiphichai, S. , Choudhury, R. , Neubauer, S. , Channon, K. M. , Torekov, S. S. , Wheelock, C. E. , & Antoniades, C. (2021). Fat‐secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. Journal of the American College of Cardiology, 77(20), 2494–2513. - PMC - PubMed
-
- Akbar, N. , Braithwaite, A. T. , Corr, E. M. , Koelwyn, G. J. , Van Solingen, C. , Cochain, C. , Saliba, A. ‐E. , Corbin, A. , Pezzolla, D. , Jørgensen, M. M. , Bæk, R. , Edgar, L. , De Villiers, C. , Gunadasa‐Rohling, M. , Banerjee, A. , Paget, D. , Lee, C. , Hogg, E. , Costin, A. , … Choudhury, R. P. (2022). Rapid neutrophil mobilisation by VCAM‐1+ endothelial extracellular vesicles. Cardiovascular Research, cvac012. 10.1093/cvr/cvac012 - DOI - PMC - PubMed
-
- Akbar, N. , Digby, J. E. , Cahill, T. J. , Tavare, A. N. , Corbin, A. L. , Saluja, S. , Dawkins, S. , Edgar, L. , Rawlings, N. , Ziberna, K. , Mcneill, E. , Johnson, E. , Aljabali, A. A. , Dragovic, R. A. , Rohling, M. , Belgard, T. G. , Udalova, I. A. , Greaves, D. R. , Channon, K. M. , & Choudhury, R. P. (2017). Endothelium‐derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. The Journal of Clinical Investigation, 2(17), e93344. - PMC - PubMed
-
- Askeland, A. , Borup, A. , Østergaard, O. , Olsen, J. V. , Lund, S. M. , Christiansen, G. , Kristensen, S. R. , Heegaard, N. H. H. , & Pedersen, S. (2020). Mass‐spectrometry based proteome comparison of extracellular vesicle isolation methods: Comparison of ME‐kit, size‐exclusion chromatography, and high‐speed centrifugation. Biomedicines, 8(8), 246. - PMC - PubMed
-
- Bæk, R. , & Jørgensen, M. M. (2017). Multiplexed phenotyping of small extracellular vesicles using protein microarray (EV Array). Methods in Molecular Biology, 1545, 117–127. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
