Antiarrhythmic Mechanisms of Epidural Blockade After Myocardial Infarction
- PMID: 38939925
- PMCID: PMC11257785
- DOI: 10.1161/CIRCRESAHA.123.324058
Antiarrhythmic Mechanisms of Epidural Blockade After Myocardial Infarction
Abstract
Background: Thoracic epidural anesthesia (TEA) has been shown to reduce the burden of ventricular tachycardia in small case series of patients with refractory ventricular tachyarrhythmias and cardiomyopathy. However, its electrophysiological and autonomic effects in diseased hearts remain unclear, and its use after myocardial infarction is limited by concerns for potential right ventricular dysfunction.
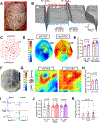
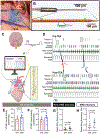
Methods: Myocardial infarction was created in Yorkshire pigs (N=22) by left anterior descending coronary artery occlusion. Approximately, six weeks after myocardial infarction, an epidural catheter was placed at the C7-T1 vertebral level for injection of 2% lidocaine. Right and left ventricular hemodynamics were recorded using Millar pressure-conductance catheters, and ventricular activation recovery intervals (ARIs), a surrogate of action potential durations, by a 56-electrode sock and 64-electrode basket catheter. Hemodynamics and ARIs, baroreflex sensitivity and intrinsic cardiac neural activity, and ventricular effective refractory periods and slope of restitution (Smax) were assessed before and after TEA. Ventricular tachyarrhythmia inducibility was assessed by programmed electrical stimulation.
Results: TEA reduced inducibility of ventricular tachyarrhythmias by 70%. TEA did not affect right ventricular-systolic pressure or contractility, although left ventricular-systolic pressure and contractility decreased modestly. Global and regional ventricular ARIs increased, including in scar and border zone regions post-TEA. TEA reduced ARI dispersion specifically in border zone regions. Ventricular effective refractory periods prolonged significantly at critical sites of arrhythmogenesis, and Smax was reduced. Interestingly, TEA significantly improved cardiac vagal function, as measured by both baroreflex sensitivity and intrinsic cardiac neural activity.
Conclusions: TEA does not compromise right ventricular function in infarcted hearts. Its antiarrhythmic mechanisms are mediated by increases in ventricular effective refractory period and ARIs, decreases in Smax, and reductions in border zone electrophysiological heterogeneities. TEA improves parasympathetic function, which may independently underlie some of its observed antiarrhythmic mechanisms. This study provides novel insights into the antiarrhythmic mechanisms of TEA while highlighting its applicability to the clinical setting.
Keywords: anesthesia, epidural; arrhythmias, cardiac; autonomic nervous system; cardiac electrophysiology; myocardial infarction; peripheral nervous system; translational research.
Conflict of interest statement
M. Vaseghi has patents related to neuromodulation at the University of California, Los Angeles, and has performed educational consulting for Biosense Webster, Medtronic, and Recor, Inc, and has shares in NeuCures and Anumana, Inc. The other authors report no conflicts.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

