A longitudinal study on SARS-CoV-2 seroconversion, reinfection and neutralisation spanning several variant waves and vaccination campaigns, Heinsberg, Germany, April 2020 to November 2022
- PMID: 38940003
- PMCID: PMC11212458
- DOI: 10.2807/1560-7917.ES.2024.29.26.2300659
A longitudinal study on SARS-CoV-2 seroconversion, reinfection and neutralisation spanning several variant waves and vaccination campaigns, Heinsberg, Germany, April 2020 to November 2022
Abstract
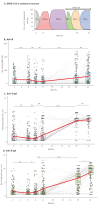
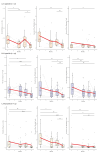
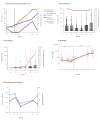
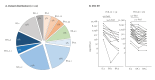
BackgroundSince its emergence in December 2019, over 700 million people worldwide have been infected with SARS-CoV-2 up to May 2024. While early rollout of mRNA vaccines against COVID-19 has saved many lives, there was increasing immune escape of new virus variants. Longitudinal monitoring of population-wide SARS-CoV-2 antibody responses from regular sample collection irrespective of symptoms provides representative data on infection and seroconversion/seroreversion rates.AimTo examine adaptive and cellular immune responses of a German SARS-CoV-2 outbreak cohort through several waves of infection with different virus variants.MethodsUtilising a 31-month longitudinal seroepidemiological study (n = 1,446; mean age: 50 years, range: 2-103) initiated during the first SARS-CoV-2 superspreading event (February 2020) in Heinsberg, Germany, we analysed acute infection, seroconversion and virus neutralisation at five follow-up visits between October 2020 and November 2022; cellular and cross-protective immunity against SARS-CoV-2 Omicron variants were also examined.ResultsSARS-CoV-2 spike (S)-specific IgAs decreased shortly after infection, while IgGs remained stable. Both increased significantly after vaccination. We predict an 18-month half-life of S IgGs upon infection. Nucleocapsid (N)-specific responses declined over 12 months post-infection but increased (p < 0.0001) during Omicron. Frequencies of SARS-CoV-2-specific TNF-alpha+/IFN-gamma+ CD4+ T-cells declined over 12 months after infection (p < 0.01). SARS-CoV-2 S antibodies and neutralisation titres were highest in triple-vaccinated participants infected between April 2021 and November 2022 compared with infections between April 2020 and January 2021. Cross neutralisation against Omicron BQ.1.18 and XBB.1.5 was very low in all groups.ConclusionInfection and/or vaccination did not provide the population with cross-protection against Omicron variants.
Keywords: BA.1; BA.2; BA.5; BQ.1.1; COVID-19; Omicron; SARS-CoV-2; XBB1.5; cohort study; long-term immunity; pandemic preparedness; seroconversion.
Conflict of interest statement
Figures

Similar articles
-
Differential COVID-19 case ascertainment by age and vaccination status in Victoria, Australia: a serosurveillance and record linkage study.Commun Dis Intell (2018). 2024 Aug 21;48. doi: 10.33321/cdi.2024.48.28. Commun Dis Intell (2018). 2024. PMID: 39165016
-
Evaluation of cross-neutralizing immunity following COVID-19 primary series vaccination during the Omicron surge in Tanzania.J Med Virol. 2024 Aug;96(8):e29822. doi: 10.1002/jmv.29822. J Med Virol. 2024. PMID: 39056238
-
Characterization of the SARS-CoV-2 antibody landscape in Norway in the late summer of 2022: high seroprevalence in all age groups with patterns of primary Omicron infection in children and hybrid immunity in adults.BMC Infect Dis. 2024 Aug 20;24(1):841. doi: 10.1186/s12879-024-09670-w. BMC Infect Dis. 2024. PMID: 39164637 Free PMC article.
-
Meta-analysis of hybrid immunity to mitigate the risk of Omicron variant reinfection.Front Public Health. 2024 Aug 26;12:1457266. doi: 10.3389/fpubh.2024.1457266. eCollection 2024. Front Public Health. 2024. PMID: 39253287 Free PMC article.
-
Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection.Nat Rev Immunol. 2021 Jun;21(6):395-404. doi: 10.1038/s41577-021-00550-x. Epub 2021 Apr 29. Nat Rev Immunol. 2021. PMID: 33927374 Free PMC article. Review.
Cited by
-
Long-Term Dynamics of SARS-CoV-2 Variant-Specific Neutralizing Antibodies Following mRNA Vaccination and Infection.Viruses. 2025 May 6;17(5):675. doi: 10.3390/v17050675. Viruses. 2025. PMID: 40431687 Free PMC article.
-
Antibody-dependent enhancement of coronaviruses.Int J Biol Sci. 2025 Feb 3;21(4):1686-1704. doi: 10.7150/ijbs.96112. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990674 Free PMC article. Review.
References
-
- Prasad N, Bansal SB, Yadav B, Manhas N, Yadav D, Gautam S, et al. Seroconversion rate after SARS-CoV-2 infection and two doses of either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ vaccination in renal allograft recipients: an experience of two public and private tertiary care center. Front Immunol. 2022;13:911738. 10.3389/fimmu.2022.911738 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
