Association of Atrial Fibrillation Burden and Mortality Among Patients With Cardiac Implantable Electronic Devices
- PMID: 38940005
- PMCID: PMC11286354
- DOI: 10.1161/CIRCULATIONAHA.124.069757
Association of Atrial Fibrillation Burden and Mortality Among Patients With Cardiac Implantable Electronic Devices
Abstract
Background: Current estimates of atrial fibrillation (AF)-associated mortality rely on claims- or clinical-derived diagnoses of AF, limit AF to a binary entity, or are confounded by comorbidities. The objective of the present study is to assess the association between device-recognized AF and mortality among patients with cardiac implantable electronic devices capable of sensitive and continuous atrial arrhythmia detection. Secondary outcomes include relative mortality among cohorts with no AF, paroxysmal AF, persistent AF, and permanent AF.
Methods: Using the deidentified Optum Clinformatics US claims database (2015 to 2020) linked to the Medtronic CareLink database, we identified individuals with a cardiac implantable electronic device who transmitted data ≥6 months after implantation. AF burden was assessed during the first 6 months after implantation (baseline period). Subsequent mortality, assessed from claims data, was compared between patients with and without AF, with adjustment for age, geographic region, insurance type, Charlson Comorbidity Index, and implantation year.
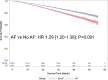
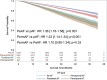
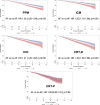
Results: Of 21 391 patients (age, 72.9±10.9 years; 56.3% male) analyzed, 7798 (36.5%) had device-recognized AF. During a mean of 22.4±12.9 months (median, 20.1 [12.8-29.7] months) of follow-up, the overall incidence of mortality was 13.5%. Patients with AF had higher adjusted all-cause mortality than patients without AF (hazard ratio, 1.29 [95% CI, 1.20-1.39]; P<0.001). Among those with AF, patients with nonparoxysmal AF had the greatest risk of mortality (persistent AF versus paroxysmal AF: hazard ratio, 1.36 [95% CI, 1.18-1.58]; P<.001; permanent AF versus paroxysmal AF: hazard ratio, 1.23 [95% CI, 1.14-1.34]; P<.001).
Conclusions: After adjustment for potential confounding factors, the presence of AF was associated with higher mortality in our cohort of patients with cardiac implantable electronic devices. Among those with AF, nonparoxysmal AF was associated with the greatest risk of mortality.
Keywords: atrial fibrillation; cardiac implantable electronic devices; mortality.
Conflict of interest statement
Dr Passman received research support from the American Heart Association (No. 18SFRN34250013) and the National Institutes of Health (UG3HL165065); research support and speaker fees from Medtronic; research support from Abbott; and royalties from UpToDate. Dr Zhou, S.C. Rosemas, and Drs Soderlund and Longacre are employees and shareholders of Medtronic. A.I. Roberts is a contractor with Medtronic. Northwestern University receives fellowship support from Medtronic. The remaining authors report no conflicts.
Figures

References
-
- Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, et al. ; Peer Review Committee Members. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193 - PMC - PubMed
-
- Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–248. doi: 10.1016/j.jval.2011.09.009 - PubMed
-
- Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck KH, Lebedev D, Rieger G, Purerfellner H; XPECT Trial Investigators. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3:141–147. doi: 10.1161/CIRCEP.109.877852 - PubMed
-
- Passman RS, Weinberg KM, Freher M, Denes P, Schaechter A, Goldberger JJ, Kadish AH. Accuracy of mode switch algorithms for detection of atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 2004;15:773–777. doi: 10.1046/j.1540-8167.2004.03537.x - PubMed
-
- Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

