Peripherally inserted central catheter design and material for reducing catheter failure and complications
- PMID: 38940297
- PMCID: PMC11212118
- DOI: 10.1002/14651858.CD013366.pub2
Peripherally inserted central catheter design and material for reducing catheter failure and complications
Abstract
Background: Peripherally inserted central catheters (PICCs) facilitate diagnostic and therapeutic interventions in health care. PICCs can fail due to infective and non-infective complications, which PICC materials and design may contribute to, leading to negative sequelae for patients and healthcare systems.
Objectives: To assess the effectiveness of PICC material and design in reducing catheter failure and complications.
Search methods: The University of Queensland and Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases and the WHO ICTRP and ClinicalTrials.gov trials registers to 16 May 2023. We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta-analyses, and health technology assessment reports. We contacted experts in the field to ascertain additional relevant information.
Selection criteria: We included randomised controlled trials (RCTs) evaluating PICC design and materials.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were venous thromboembolism (VTE), PICC-associated bloodstream infection (BSI), occlusion, and all-cause mortality. Secondary outcomes were catheter failure, PICC-related BSI, catheter breakage, PICC dwell time, and safety endpoints. We assessed the certainty of evidence using GRADE.
Main results: We included 12 RCTs involving approximately 2913 participants (one multi-arm study). All studies except one had a high risk of bias in one or more risk of bias domain. Integrated valve technology compared to no valve technology for peripherally inserted central catheter design Integrated valve technology may make little or no difference to VTE risk when compared with PICCs with no valve (risk ratio (RR) 0.71, 95% confidence interval (CI) 0.19 to 2.63; I² = 0%; 3 studies; 437 participants; low certainty evidence). We are uncertain whether integrated valve technology reduces PICC-associated BSI risk, as the certainty of the evidence is very low (RR 0.20, 95% CI 0.01 to 4.00; I² = not applicable; 2 studies (no events in 1 study); 257 participants). Integrated valve technology may make little or no difference to occlusion risk when compared with PICCs with no valve (RR 0.86, 95% CI 0.53 to 1.38; I² = 0%; 5 studies; 900 participants; low certainty evidence). We are uncertain whether use of integrated valve technology reduces all-cause mortality risk, as the certainty of evidence is very low (RR 0.85, 95% CI 0.44 to 1.64; I² = 0%; 2 studies; 473 participants). Integrated valve technology may make little or no difference to catheter failure risk when compared with PICCs with no valve (RR 0.80, 95% CI 0.62 to 1.03; I² = 0%; 4 studies; 720 participants; low certainty evidence). We are uncertain whether integrated-valve technology reduces PICC-related BSI risk (RR 0.51, 95% CI 0.19 to 1.32; I² = not applicable; 2 studies (no events in 1 study); 542 participants) or catheter breakage, as the certainty of evidence is very low (RR 1.05, 95% CI 0.22 to 5.06; I² = 20%; 4 studies; 799 participants). Anti-thrombogenic surface modification compared to no anti-thrombogenic surface modification for peripherally inserted central catheter design We are uncertain whether use of anti-thrombogenic surface modified catheters reduces risk of VTE (RR 0.67, 95% CI 0.13 to 3.54; I² = 15%; 2 studies; 257 participants) or PICC-associated BSI, as the certainty of evidence is very low (RR 0.20, 95% CI 0.01 to 4.00; I² = not applicable; 2 studies (no events in 1 study); 257 participants). We are uncertain whether use of anti-thrombogenic surface modified catheters reduces occlusion (RR 0.69, 95% CI 0.04 to 11.22; I² = 70%; 2 studies; 257 participants) or all-cause mortality risk, as the certainty of evidence is very low (RR 0.49, 95% CI 0.05 to 5.26; I² = not applicable; 1 study; 111 participants). Use of anti-thrombogenic surface modified catheters may make little or no difference to risk of catheter failure (RR 0.76, 95% CI 0.37 to 1.54; I² = 46%; 2 studies; 257 participants; low certainty evidence). No PICC-related BSIs were reported in one study (111 participants). As such, we are uncertain whether use of anti-thrombogenic surface modified catheters reduces PICC-related BSI risk (RR not estimable; I² = not applicable; very low certainty evidence). We are uncertain whether use of anti-thrombogenic surface modified catheters reduces the risk of catheter breakage, as the certainty of evidence is very low (RR 0.15, 95% CI 0.01 to 2.79; I² = not applicable; 2 studies (no events in 1 study); 257 participants). Antimicrobial impregnation compared to non-antimicrobial impregnation for peripherally inserted central catheter design We are uncertain whether use of antimicrobial-impregnated catheters reduces VTE risk (RR 0.54, 95% CI 0.05 to 5.88; I² = not applicable; 1 study; 167 participants) or PICC-associated BSI risk, as the certainty of evidence is very low (RR 2.17, 95% CI 0.20 to 23.53; I² = not applicable; 1 study; 167 participants). Antimicrobial-impregnated catheters probably make little or no difference to occlusion risk (RR 1.00, 95% CI 0.57 to 1.74; I² = 0%; 2 studies; 1025 participants; moderate certainty evidence) or all-cause mortality (RR 1.12, 95% CI 0.71 to 1.75; I² = 0%; 2 studies; 1082 participants; moderate certainty evidence). Antimicrobial-impregnated catheters may make little or no difference to risk of catheter failure (RR 1.04, 95% CI 0.82 to 1.30; I² = not applicable; 1 study; 221 participants; low certainty evidence). Antimicrobial-impregnated catheters probably make little or no difference to PICC-related BSI risk (RR 1.05, 95% CI 0.71 to 1.55; I² = not applicable; 2 studies (no events in 1 study); 1082 participants; moderate certainty evidence). Antimicrobial-impregnated catheters may make little or no difference to risk of catheter breakage (RR 0.86, 95% CI 0.19 to 3.83; I² = not applicable; 1 study; 804 participants; low certainty evidence).
Authors' conclusions: There is limited high-quality RCT evidence available to inform clinician decision-making for PICC materials and design. Limitations of the current evidence include small sample sizes, infrequent events, and risk of bias. There may be little to no difference in the risk of VTE, PICC-associated BSI, occlusion, or mortality across PICC materials and designs. Further rigorous RCTs are needed to reduce uncertainty.
Trial registration: ClinicalTrials.gov NCT00621712.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JAS: none known.
TK: The University of Queensland has received investigator‐initiated research grants unrelated to the current study from BD‐Bard and 3M. Over five years ago, AngioDynamics provided funding to TK's former employer (Griffith University) for part of a randomised controlled trial that was eligible for inclusion in this Cochrane review. This grant was investigator‐initiated, and AngioDynamics did not play any part in study development, data collection, analysis, or decision to publish. TK did not review this study. The review of this study was undertaken by other authors of the review. All potential conflicts of interest have been declared in the statements above. TK declares that she does not have any additional conflicts of interest. TK declares that all payments have been made to her institution, and she has not received any personal gain from these bodies and none of the declared funding will bias the review.
KC: none known.
EY: none known.
AU: The University of Queensland has received investigator‐initiated research grants unrelated to the current study from BD‐Bard and 3M. Over five years ago, AngioDynamics provided funding to AU's former employer (Griffith University) for part of a randomised controlled trial that was eligible for inclusion in this Cochrane review. This grant was investigator‐initiated, and AngioDynamics did not play any part in study development, data collection, analysis, or decision to publish. AU did not review this study. The review of this study was undertaken by other authors of the review. All potential conflicts of interest have been declared in the statements above. AU declares that she does not have any additional conflicts of interest. AU declares that all payments have been made to her institution, and she has not received any personal gain from these bodies and none of the declared funding will bias the review.
Figures
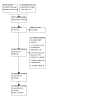

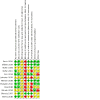


































































Update of
References
References to studies included in this review
Gavin 2020 {published data only}
Gilbert 2019 {published data only (unpublished sought but not used)}
-
- Gilbert R, Brown M, Rainford N, Donohue C, Fraser C, Sinha A, et al. Antimicrobial-impregnated central venous catheters for prevention of neonatal bloodstream infection (PREVAIL): an open-label, parallel-group, pragmatic, randomised controlled trial. Lancet Child and Adolescent Health 2019;3(6):381-90. - PubMed
Hoffer 1999 {published data only (unpublished sought but not used)}
-
- Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR. American Journal of Roentgenology 1999;173(5):1393-8. - PubMed
Hoffer 2001 {published data only (unpublished sought but not used)}
-
- Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. Journal of Vascular and Interventional Radiology 2001;12(10):1173-7. - PubMed
Itkin 2014 {published data only}
-
- Itkin M, Mondshein JI, Stavropoulos SW, Shlansky-Goldberg RD, Soulen MC, Trerotola SO. Peripherally inserted central catheter thrombosis-reverse tapered versus nontapered catheters: a randomized controlled study. Journal of Vascular and Interventional Radiology 2014;25(1):85-91. - PubMed
Johnston 2012 {published data only}
-
- Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates-the 'ELeCTRiC' study. Journal of Vascular Access 2012;13(4):421-5. - PubMed
Kleidon 2018 {published data only}
-
- Kleidon T, Ullman AJ, Zhang L, Mihala G, Chaseling B, Schoutrop J, et al. How does your PICCOMPARE? A pilot randomized controlled trial comparing various PICC materials in pediatrics. Journal of Hospital Medicine 2019;13(8):517-25. - PubMed
Miyagaki 2012 {published data only}
-
- Miyagaki H, Nakajima K, Hara J, Yamasaki M, Kurokawa Y, Miyata H, et al. Performance comparison of peripherally inserted central venous catheters in gastrointestinal surgery: a randomized controlled trial. Clinical Nutrition 2012;31(1):48-52. - PubMed
Ong 2010 {published data only (unpublished sought but not used)}
-
- Ong CK, Venkatesh SK, Lau GB, Wang SC. Prospective randomized comparative evaluation of proximal valve polyurethane and distal valve silicone peripherally inserted central catheters. Journal of Vascular and Interventional Radiology 2010;21(8):1191-6. - PubMed
Pittiruti 2014 {published data only}
-
- Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. Journal of Vascular Access 2014;15(6):519-23. - PubMed
Sheretz 1997 {published data only}
-
- Sherertz RJ, Stephens JL, Marosok RD, Carruth WA, Rich HA, Hampton KD, et al. The risk of peripheral vein phlebitis associated with chlorhexidine-coated catheters: a randomized, double-blind trial. Infection Control and Hospital Epidemiology 1997;18(4):230-6. - PubMed
Storey 2016 {published data only}
-
- Storey S, Brown J, Foley A, Newkirk E, Powers J, Barger J, et al. A comparative evaluation of antimicrobial coated versus nonantimicrobial coated peripherally inserted central catheters on associated outcomes: a randomized controlled trial. American Journal of Infection Control 2016;44(6):636-41. - PubMed
References to studies excluded from this review
Alport 2012 {published data only (unpublished sought but not used)}
-
- Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: a tale of two PICCs. Canadian Association of Radiologists Journal 2012;63(4):323-8. - PubMed
Bach 1998 {published data only}
-
- Bach A. A randomized trial of an antibiotic- and antiseptic-coated central venous catheter in the prevention of catheter-related infections. Archives of Surgery 1998;133(9):1022. - PubMed
Bennegard 1982 {published data only}
-
- Bennegard K, Curelaru I, Gustavsson B, Linder LE, Zachrisson BF. Material thrombogenicity in central venous catheterization. I. A comparison between uncoated and heparin-coated, long antebrachial, polyethylene catheters. Acta Anaesthesiologica Scandinavica 1982;26(2):112-20. - PubMed
Chu 2007 {published data only}
-
- Chu FS, Cheng VC, Law MW, Tso WK. Efficacy and complications in peripherally inserted central catheter insertion: a study using 4-Fr non-valved catheters and a single infusate. Australasian Radiology 2007;51(5):453-7. - PubMed
Chu 2010 {published data only}
-
- Tsai MH, Chu SM, Lien R, Huang HR, Wang JW, Chiang CC, et al. Complications associated with two different types of percutaneously inserted central venous catheter in very low birth weight infants. Infection Control and Hospital Epidemiology 2011;32(3):258-66. - PubMed
Di Giacomo 2009 {published data only}
-
- Di Giacomo M. RETRACTED: Comparison of three peripherally-inserted central catheters: pilot study. British Journal of Nursing 2009;18(1):8-16. - PubMed
Garland 2008 {published data only}
-
- Garland JS, Alex CP, Sevallius JM, Murphy DM, Good MJ, Volberding AM, et al. Cohort study of the pathogenesis and molecular epidemiology of catheter-related bloodstream infection in neonates with peripherally inserted central venous catheters. Infection Control and Hospital Epidemiology 2008;29(3):243-9. - PubMed
Kagan 2019 {published data only}
-
- Kagan E, Salgado CD, Banks AL, Marculescu CE, Cantey JR. Peripherally inserted central catheter-associated bloodstream infection: risk factors and the role of antibiotic-impregnated catheters for prevention. American Journal of Infection Control 2019;47(2):191-5. - PubMed
Kang 2016 {published data only}
-
- Kang F, Wheeler K, Ryu R, Johnson D. Size matters, reducing peripherally inserted central venous access associated thrombosis. Journal of Vascular and Interventional Radiology 2016;3(27):S197.
Khaldi 2009 {published data only}
-
- Khalidi N, Kovacevich DS, Papke-O'Donnell LF, Btaiche I. Impact of the positive pressure valve on vascular access device occlusions and bloodstream infections. Journal of the Association for Vascular Access 2009;14(2):84-91.
Leowenthal 2014 {published data only}
-
- Loewenthal M, Dobson P, Collins N, Harbort Y, Kuzmich D, Popering G, et al. Valved versus non-valved PICCs in hospital in the home (HITH) - a multi-site national study. Journal of Vascular Access 2014;15(3):202.
Lozano 2012 {published data only}
-
- Lozano LA, Marn C, Goodman LR. Power injectable peripherally inserted central venous catheter lines frequently flip after power injection of contrast. Journal of Computer Assisted Tomography 2012;36(4):427-30. - PubMed
Mermel 2007 {published data only}
-
- Mermel LA. Prevention of central venous catheter-related infections: what works other than impregnated or coated catheters? Journal of Hospital Infection 2007;65:30-3. - PubMed
NCT00621712 {unpublished data only}
-
- NCT00621712. Clinical assessment of a new catheter surface coating with antimicrobial properties. clinicaltrials.gov/show/NCT00621712.
Parker 1995 {published data only}
-
- Parker JW, Gaines RW Jr. Long-term intravenous therapy with use of peripherally inserted silicone-elastomer catheters in orthopaedic patients. Journal of Bone and Joint Surgery 1995;77(4):572-7. - PubMed
Poli 2016 {published data only}
-
- Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabrò EM. A comparative study on the mechanical behavior of polyurethane PICCs. Journal of Vascular Access 2016;17(2):175-81. - PubMed
Ridyard 2017 {published data only}
Rupp 2005 {published data only}
-
- Rupp ME, Lisco SJ, Lipsett PA, Perl TM, Keating K, Civetta JM, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Annals of Internal Medicine 2005;143(8):570-80. - PubMed
Toh 2013 {published data only}
-
- Toh LM, Mavili E, Moineddin R, Amaral J, John PR, Temple MJ, et al. Are cuffed peripherally inserted central catheters superior to uncuffed peripherally inserted central catheters? A retrospective review in a tertiary pediatric center. Journal of Vascular and Interventional Radiology 2013;24(9):1316-22. - PubMed
Treotola 2010 {published data only}
-
- Trerotola SO, Stavropoulos SW, Mondschein JI, Patel AA, Fishman N, Fuchs B, et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology 2010;256(1):312-20. - PubMed
van Vliet 2001 {published data only}
-
- Vliet JV, Leusink JA, Jongh BD, Boer AD. A comparison between two types of central venous catheters in the prevention of catheter-related infections: the importance of performing all the relevant cultures. Clinical Intensive Care 2001;12(3):135-40.
Wheeler 1992 {published data only}
-
- Wheeler RA, Malone PS. Prospective trial of silicone and polyurethane long-term central lines in children: a requiem for silicone. Pediatric Surgery International 1992;7:468-70.
Wu 2019 {published data only}
Yang 2012 {published data only}
-
- Yang RY, Moineddin R, Filipescu D, Parra D, Amaral J, John P, et al. Increased complexity and complications associated with multiple peripherally inserted central catheter insertions in children: the tip of the iceberg. Journal of Vascular and Interventional Radiology 2012;23(3):351-7. - PubMed
References to studies awaiting assessment
Cassim 2019 {published data only (unpublished sought but not used)}
-
- Cassim CR, Boucher LM, Paquet F, Valenti DA. To demonstrate whether the claim that using the CHLORAG+ARD© impregnated PICC decreases incidence of CLABSI and CR-UEDVT verses polyurethane power injectable (BARD) PICC in oncology patients, to justify its increased price. In: Cardiovascular and Interventional Radiological Society of Europe. Barcelona, Spain, 2019:1.
Yoon 2016 {published and unpublished data}
-
- Yoon H, Drabkin M, Loya M, Patel C, Saif A, Shah S. Prospective randomized evaluation of complications with Endexo PICC Technology (PRECEPT). Journal of Vascular and Interventional Radiology 2016;3:S283.
References to ongoing studies
ACTRN12616001354471 {published data only}
-
- ACTRN12616001354471. Comparing the effectiveness of PowerPICC with BioFlo on peripherally-inserted central catheter (PICC)-related occlusion and infection rates in the oncology/haematology setting: a randomised controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12616001354471 (first received 29 September 2016).
ACTRN12619000022167 {unpublished data only}
-
- ACTRN12619000022167. Randomised controlled trial in adults and children of anti-thrombogenic PICCs, and antiseptic PICCs, in comparison to polyurethane PICCs (standard care), to prevent PICC failure and complications. anzctr.org.au/ACTRN12619000022167.aspx.
ChiCTR2000030971 {published data only}
-
- ChiCTR2000030971. A comparative study of the incidence of occlusion in two different types of PICC catheters in outpatients. who.int/Trial2.aspx?TrialID=ChiCTR2000030971.
NCT05278507 {published data only}
-
- NCT05278507. Comparing Arrow PICC Catheters w/ Arrowga+rd Blue Advanced Protection Performance and Safety to unprotected PICC's. clinicaltrials.gov/study/NCT05278507 (first received 21 February 2022).
RBR‐48342g {unpublished data only}
-
- RBR-48342g. Comparison of catheters placed in the vein regarding the formation of blood clots. trialsearch.who.int/Trial2.aspx?TrialID=RBR-48342g (first received 18 April 2019).
Additional references
Abedin 2008
-
- Abedin S, Kapoor G. Peripherally inserted central venous catheters are a good option for prolonged venous access in children with cancer. Pediatric Blood & Cancer 2008;51(2):251-5. - PubMed
Al‐Asadi 2019
-
- Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thrombosis Journal 2019;17:1-7. [DOI: 10.1186/a12959-019-0191-y] - DOI - PMC - PubMed
Bertoglio 2016
-
- Bertoglio S, Faccini B, Lalli L, Cafiero F, Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. Journal of Surgical Oncology 2016;113(6):708-14. - PubMed
Chopra 2012
-
- Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. American Journal of Medicine 2012;125(8):733-41. - PubMed
Chopra 2013a
-
- Chopra V, Kuhn L, Coffey Jr CE, Salameh M, Barron J, Krein S, et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. Journal of Hospital Medicine 2013;8(6):309-14. - PubMed
Chopra 2013b
-
- Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 2013;382(9889):311-25. - PubMed
Chopra 2013c
-
- Chopra V, O'Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infection Control and Hospital Epidemiology 2013;34(9):908-18. - PubMed
Chopra 2014
-
- Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. American Journal of Medicine 2014;127(4):319-28. - PubMed
Chopra 2022
-
- Chopra V, O'Malley M, Horowitz J, Zhang Q, McLaughlin E, Saint S, et al. Improving peripherally inserted central catheter appropriateness and reducing device-related complications: a quasiexperimental study in 52 Michigan hospitals. BMJ Safety and Quality 2022;31(1):23-30. [DOI: 10.1136/bmjqs-2021-013015] - DOI - PMC - PubMed
DeVries 2021
-
- DeVries M, Sleweon T. Bridging the gap: introduction of an antimicrobial peripherally inserted central catheter (PICC) in response to high PICC central line-associated bloodstream infection incidence. British Journal of Nusing 2021;30(19 Suppl 1):16-22. [DOI: 10.12968/bjon.2021.30.19.S16] - DOI - PubMed
Di Carlo 2012
-
- Di Carlo I, Biffi R. Total Implantable Venous Access Devices. New York (NY): Springer, 2012.
Freitas 2003
-
- Freitas RA. Nanomedicine, Volume IIA: Biocompatibility. 1st edition. Georgetown (TX): Milton: CRC Press, 2003.
Frey 2006
-
- Frey AM, Schears GJ. Why are we stuck on tape and suture?: a review of catheter securement devices. Journal of Infusion Nursing 2006;29(1):34-8. - PubMed
Gallieni 2008
-
- Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA: A Cancer Journal for Clinicians 2008;58(6):323-46. - PubMed
Gnannt 2018
-
- Gnannt R, Waespe N, Temple M, Amirabadi A, Liu K, Brandao LR, et al. Increased risk of symptomatic upper-extremity venous thrombosis with multiple peripherally inserted central catheter insertions in pediatric patients. Pediatric Radiology 2018;48:1013-20. - PubMed
Goossens 2016
-
- Goossens GA, Waele YD, Jerome M, Fieuws S, Janssens C, Stas M, et al. Diagnostic accuracy of the Catheter Injection and Aspiration (CINAS) classification for assessing the function of totally implantable venous access devices. Supportive Cancer in Care 2016;24:755-61. - PubMed
GRADE Handbook 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADE Working Group
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 21 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Churchill R, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Database of Systematic Reviews of Interventions 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2023
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hoffer 2001
-
- Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. Journal of Vascular and Interventional Radiology 2001;12(10):1173-7. - PubMed
Horan 2008
-
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309-32. - PubMed
Johansson 2013
-
- Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncologica 2013;52(5):886-92. - PubMed
Kleidon 2018
-
- Kleidon T, Ullman AJ, Zhang L, Mihala G, Chaseling B, Schoutrop J, et al. How does your PICCOMPARE? A pilot randomized controlled trial comparing various PICC materials in pediatrics. Journal of Hospital Medicine 2018;13(8):517-25. - PubMed
Kramer 2015
-
- Kramer R, Conte M, Mann J, Saint S, Chopra V. Do antimicrobial peripherally inserted central catheters reduce the risk of central line-associated bloodstream infection? A systematic review and meta-analysis. Open Forum Infectious Diseases 2015;2 Suppl 1:283.
Lai 2016
-
- Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD007878. [DOI: 10.1002/14651858.CD007878.pub3] - DOI - PMC - PubMed
Lefebvre 2023
-
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Metzendorf M, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):W-65. - PubMed
May 2015
Mermel 2001
-
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Journal of Intravenous Nursing 2001;24(3):180-205. - PubMed
Mermel 2009
-
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;49(1):1-45. [DOI: 10.1086/599376] - DOI - PMC - PubMed
Moher 2009
Periard 2008
-
- Periard D, Monney P, Waeber G, Zurkinden C, Mazzolai L, Hayoz D, et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. Journal of Thrombosis and Haemostasis 2008;6(8):1281-8. - PubMed
Pittiruti 2014
-
- Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. Journal of Vascular Access 2014;15(6):519-23. - PubMed
Poli 2016
-
- Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. Journal of Vascular Access 2016;17(2):175-81. - PubMed
Raad 2009
-
- Raad I, Kassar R, Ghannam D, Chaftari AM, Hachem R, Jiang Y. Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteraemia: remove or retain? Clinical Infectious Diseases 2009;49(8):1187-94. - PubMed
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.2.0. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
Russell 2014
-
- Russell E, Chan R, Marsh N, New K. A point prevalence study of cancer nursing practices for managing intravascular devices in an Australian tertiary cancer center. European Journal of Oncology Nursing 2014;18(3):231-5. - PubMed
Schults 2021
Schults 2022
Schünemann 2023
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Seckold 2015
-
- Seckold T, Walker S, Dwyer T. A comparison of silicone and polyurethane PICC lines and postinsertion complication rates: a systematic review. Journal of Vascular Access 2015;16(3):167-77. - PubMed
Shalabi 2015
-
- Shalabi M, Adel M, Yoon E, Aziz K, Lee S, Shah PS, Canadian Neonatal Network. Risk of infection using peripherally inserted central and umbilical catheters in preterm neonates. Pediatrics 2015;136(6):1073-9. - PubMed
Shen 2009
-
- Shen G, Gao Y, Wang Y, Mao B, Wang X. Survey of the long-term use of peripherally inserted central venous catheters in children with cancer: experience in a developing country. Journal of Pediatric Hematology/Oncology 2009;31(7):489-92. - PubMed
Slaughter 2020
-
- Slaughter E, Kynoch K, Brodribb M, Keogh SJ. Evaluating the impact of central venous catheter materials and design on thrombosis: a systematic review and meta-analysis. Worldviews on Evidence-Based Nursing 2020;17(5):376-84. - PubMed
Ullman 2015
-
- Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics 2015;136(5):e1331-44. - PubMed
Ullman 2017
-
- Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. Journal of Paediatrics and Child Health 2017;53(2):123-30. - PubMed
Ullman 2018
-
- Ullman AJ, Bulmer AC, Dargaville TR, Rickard CM, Chopra V. Antithrombogenic peripherally inserted central catheters: overview of efficacy and safety. Expert Review of Medical Devices 2019;16(1):25-33. - PubMed
Ullman 2022a
-
- Ullman AJ, Paterson RS, Schults JA, Kleidon TM, August D, O'Malley M, et al. Do antimicrobial and antithrombogenic peripherally inserted central catheter (PICC) materials prevent catheter complications? an analysis of 42,562 hospitalized medical patients. Infection Control and Hospital Epidemiology 2022;43(4):427-34. - PubMed
Ullman 2022b
Ullman 2023
Warren 2006
-
- Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a non-teaching hospital. Critical Care Medicine 2006;34(8):2084-9. - PubMed
Wilson 2014
-
- Wilson MZ, Rafferty C, Deeter D, Comito MA, Hollenbeak CS. Attributable costs of central line-associated bloodstream infections in a pediatric hematology/oncology population. American Journal of Infection Control 2014;42(11):1157-60. - PubMed
Yamamoto 2002
-
- Yamamoto AJ, Solomon JA, Soulen MC, Tang J, Parkinson K, Lin R, et al. Sutureless securement device reduces complications of peripherally inserted central venous catheters. Journal of Vascular and Interventional Radiology 2002;13(1):77-81. - PubMed
Yang 2012
-
- Yang RY, Moineddin R, Filipescu D, Parra D, Amaral J, John P, et al. Increased complexity and complications associated with multiple peripherally inserted central catheter insertions in children: the tip of the iceberg. Journal of Vascular and Interventional Radiology 2012;23(3):351-7. - PubMed
Yap 2006
-
- Yap YS, Karapetis C, Lerose S, Iyer S, Koczwara B. Reducing the risk of peripherally inserted central catheter line complications in the oncology setting. European Journal of Cancer Care 2006;15(4):342-7. - PubMed
Ziegler 2015
-
- Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection 2015;43:29-36. - PubMed
References to other published versions of this review
Schults 2019
-
- Schults JA, Kleidon T, Petsky HL, Stone R, Schoutrop J, Ullman AJ. Peripherally inserted central catheter design and material for reducing catheter failure and complications. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD013366. [DOI: 10.1002/14651858.CD013366] - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

