Association of glial fibrillary acid protein, Alzheimer's disease pathology and cognitive decline
- PMID: 38940331
- PMCID: PMC11629700
- DOI: 10.1093/brain/awae211
Association of glial fibrillary acid protein, Alzheimer's disease pathology and cognitive decline
Abstract
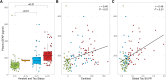
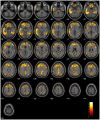
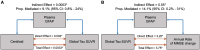
Increasing evidence shows that neuroinflammation is a possible modulator of tau spread effects on cognitive impairment in Alzheimer's disease. In this context, plasma levels of the glial fibrillary acidic protein (GFAP) have been suggested to have a robust association with Alzheimer's disease pathophysiology. This study aims to assess the correlation between plasma GFAP and Alzheimer's disease pathology, and their synergistic effect on cognitive performance and decline. A cohort of 122 memory clinic subjects with amyloid and tau PET, MRI scans, plasma GFAP and Mini-Mental State Examination (MMSE) was included in the study. A subsample of 94 subjects had a follow-up MMSE score at ≥1 year after baseline. Regional and voxel-based correlations between Alzheimer's disease biomarkers and plasma GFAP were assessed. Mediation analyses were performed to evaluate the effects of plasma GFAP on the association between amyloid and tau PET and between tau PET and cognitive impairment and decline. GFAP was associated with increased tau PET ligand uptake in the lateral temporal and inferior temporal lobes in a strong left-sided pattern independently of age, sex, education, amyloid and APOE status (β = 0.001, P < 0.01). The annual rate of MMSE change was significantly and independently correlated with both GFAP (β = 0.006, P < 0.01) and global tau standardized uptake value ratio (β = 4.33, P < 0.01), but not with amyloid burden. Partial mediation effects of GFAP were found on the association between amyloid and tau pathology (13.7%) and between tau pathology and cognitive decline (17.4%), but not on global cognition at baseline. Neuroinflammation measured by circulating GFAP is independently associated with tau Alzheimer's disease pathology and with cognitive decline, suggesting neuroinflammation as a potential target for future disease-modifying trials targeting tau pathology.
Keywords: Alzheimer’s disease biomarkers; cognitive decline; glial fibrillary acidic protein; neurofibrillary tau tangles; positron emission tomography.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
V.G. received research support and speaker fees through her institution from GE Healthcare, Siemens Healthineers and Novo Nordisk. G.B.F. has received support, payment, consulting fees or honoraria through his institution for lectures, presentations, speaker bureaus, manuscript writing or educational events from: Biogen, Roche, Diadem, Novo Nordisk, GE Healthcare, OM Pharma and Eisai. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programmes for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. The other authors have no conflicts of interest to disclose.
Figures
Comment in
-
Astroglial reactivity is a key modulator of Alzheimer's disease pathological progression.Brain. 2024 Dec 3;147(12):3973-3975. doi: 10.1093/brain/awae354. Brain. 2024. PMID: 39514769 Free PMC article.
References
-
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. - PubMed
-
- Khoury R, Ghossoub E. Diagnostic biomarkers of Alzheimer’s disease: A state-of-the-art review. Biomark Neuropsychiatry. 2019;1:100005.
-
- Chételat G, Arbizu J, Barthel H, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19:951–962. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

